Direct seawater electrolysis by adjusting the local reaction environment of a catalyst
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 46
Abstract
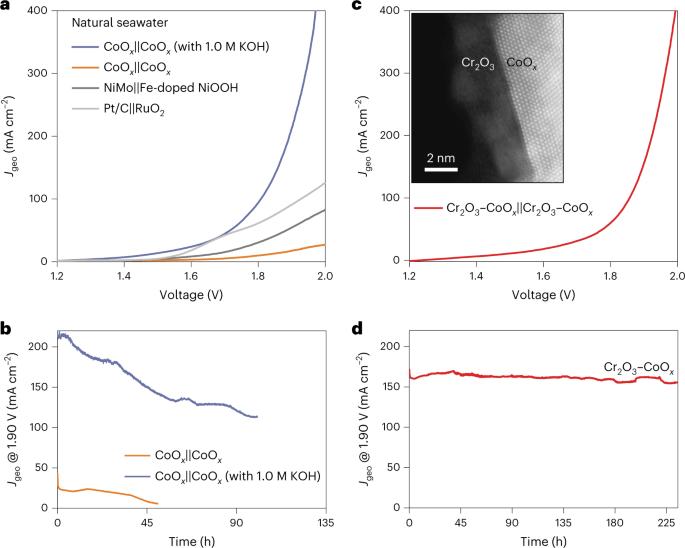
The use of vast amounts of high-purity water for hydrogen production may aggravate the shortage of freshwater resources. Seawater is abundant but must be desalinated before use in typical proton exchange membrane (PEM) electrolysers. Here we report direct electrolysis of real seawater that has not been alkalised nor acidified, achieving long-term stability exceeding 100 h at 500 mA cm−2 and similar performance to a typical PEM electrolyser operating in high-purity water. This is achieved by introducing a Lewis acid layer (for example, Cr2O3) on transition metal oxide catalysts to dynamically split water molecules and capture hydroxyl anions. Such in situ generated local alkalinity facilitates the kinetics of both electrode reactions and avoids chloride attack and precipitate formation on the electrodes. A flow-type natural seawater electrolyser with Lewis acid-modified electrodes (Cr2O3–CoOx) exhibits the industrially required current density of 1.0 A cm−2 at 1.87 V and 60 °C. Direct seawater electrolysis is an approach to produce hydrogen from an abundant water source, but current catalysts face performance and durability challenges. Here Guo et al. introduce a hard Lewis acid layer on the catalyst surface that generates local alkalinity, facilitating water splitting and minimizing degradation.
通过调节催化剂的局部反应环境直接进行海水电解
使用大量高纯度水制氢可能会加剧淡水资源的短缺。海水资源丰富,但在用于典型的质子交换膜(PEM)电解槽之前必须进行脱盐处理。在此,我们报告了未经碱化或酸化的真实海水的直接电解,在 500 mA cm-2 的条件下实现了超过 100 小时的长期稳定性,其性能与在高纯度水中运行的典型 PEM 电解槽相似。这是通过在过渡金属氧化物催化剂上引入路易斯酸层(如 Cr2O3)来动态分裂水分子并捕获羟基阴离子实现的。这种原位生成的局部碱度有利于两个电极反应的动力学,并避免了电极上氯化物的侵蚀和沉淀的形成。采用路易斯酸改性电极(Cr2O3-CoOx)的流动型天然海水电解槽在 1.87 V 和 60 °C 的电压条件下,电流密度达到了工业要求的 1.0 A cm-2。直接电解海水是利用丰富水源制氢的一种方法,但目前的催化剂在性能和耐久性方面面临挑战。在此,Guo 等人在催化剂表面引入了坚硬的路易斯酸层,可产生局部碱度,促进水的分裂并最大限度地减少降解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


