Photochemical diversification of strong C(sp3)–H bonds enabled by allyl bromide and sodium fluoride
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 4
Abstract
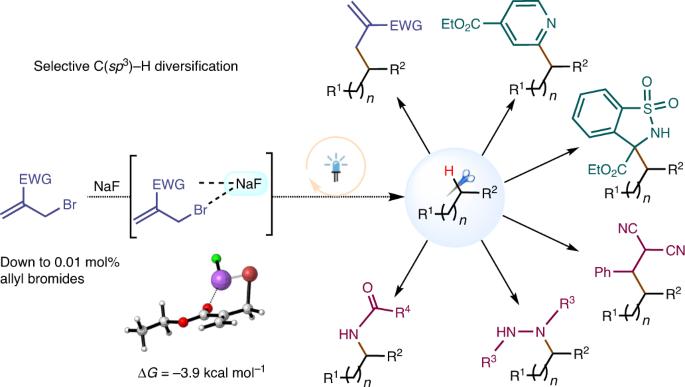
The development of practical approaches for the selective functionalization of strong, neutral C(sp3)–H bonds, such as those in petroleum-derived hydrocarbons, is of general interest but remains a remarkable challenge in synthetic chemistry. We here report a convenient approach that employs allyl bromides as the reagents and sodium fluoride as an activator in photochemical processes. Diverse C(sp3)–H functionalizations of alkanes, cycloalkanes and other relatively unreactive substances were enabled by using a stoichiometric or catalytic amount of allyl bromides as initiators in the presence of NaF, which furnished various allylated, heteroarylated, alkylated, hydrazinated and aminated products in good yields with high chemoselectivity and site selectivity. Binary NaF–allyl bromide adducts generated in situ appear to play essential roles in the reaction as light-active species, initiators for radical-mediated C–H cleavage and potential functionalization reagents. We expect that this transition-metal- and photosensitizer-free strategy will offer new opportunities for the C–H diversification of hydrocarbon feedstocks and the late-stage modification of lead compounds. The selective functionalization of strong, neutral C(sp3)–H bonds, such as in alkanes, is synthetically challenging. Now, a transition-metal- and photosensitizer-free strategy employing allyl bromides as reagents and sodium fluoride as an activator has been developed for the selective C(sp3)–H functionalization of alkanes, cycloalkanes and other relatively unreactive molecules.
烯丙基溴和氟化钠使强C(sp3) -H键光化学多样化
开发实用的方法来选择性地官能化强中性 C(sp3)-H 键(如石油衍生烃中的 C(sp3)-H 键)引起了人们的普遍兴趣,但这仍然是合成化学中的一项重大挑战。我们在此报告一种方便的方法,即在光化学过程中使用烯丙基溴作为试剂和氟化钠作为活化剂。在 NaF 的存在下,使用一定量或催化量的烯丙基溴作为引发剂,可以对烷烃、环烷烃和其他相对非活性物质进行多种 C(sp3)-H 官能化反应,生成各种烯丙基化、杂芳基化、烷基化、肼化和胺化产物,且收率高、化学选择性和位点选择性高。原位生成的二元 NaF-烯丙基溴加合物作为光活性物种、自由基介导的 C-H 裂解的引发剂和潜在的官能化试剂,似乎在反应中发挥了重要作用。我们预计,这种不含过渡金属和光敏剂的策略将为碳氢化合物原料的 C-H 多样化和先导化合物的后期改性提供新的机遇。强中性 C(sp3)-H 键(如烷烃中的 C(sp3)-H 键)的选择性官能化在合成上具有挑战性。现在,我们开发出了一种无过渡金属和光敏剂的策略,采用烯丙基溴作为试剂,氟化钠作为活化剂,对烷烃、环烷烃和其他相对非活性分子进行选择性 C(sp3)-H 功能化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


