Effect of Intramolecular Donor-Acceptor Interactions on the Radiolysis of Organic Compounds: Effects in Acetylacetone
IF 1.5
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
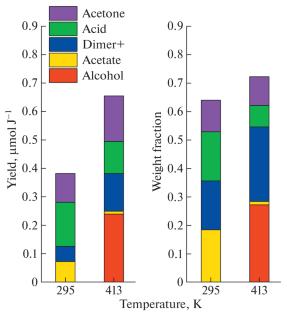
It has been shown for acetylacetone as an example that the intramolecular hydrogen bond significantly affects the radiolytic transformations of organic compounds by suppressing the proton transfer from the primary radical cation to the molecule and also by promoting cleavage of the C–OH bond in the enol form. Due to these effects, the major heavy product of radiolysis at 295 K is 4-oxopent-2-en-2-yl acetate. Under boiling conditions (413 K), hydrogen bonds are cleaved, resulting in the predominant formation of 4-hydroxy-2-pentanone, which is not detected at 295 K.
分子内供体-受体相互作用对有机化合物辐射分解的影响:对乙酰丙酮的影响
以乙酰丙酮为例,分子内氢键通过抑制质子从初级自由基阳离子向分子的转移和促进烯醇形式的C-OH键的裂解,显著影响有机化合物的辐射分解转化。由于这些影响,295k下放射性溶解的主要重产物是4-氧氧苯-2-烯-2-乙酸酯。在沸点条件下(413 K),氢键被劈裂,导致主要形成4-羟基-2-戊酮,而在295 K时没有检测到。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Doklady Physical Chemistry
化学-物理化学
CiteScore
1.50
自引率
0.00%
发文量
9
审稿时长
6-12 weeks
期刊介绍:
Doklady Physical Chemistry is a monthly journal containing English translations of current Russian research in physical chemistry from the Physical Chemistry sections of the Doklady Akademii Nauk (Proceedings of the Russian Academy of Sciences). The journal publishes the most significant new research in physical chemistry being done in Russia, thus ensuring its scientific priority. Doklady Physical Chemistry presents short preliminary accounts of the application of the state-of-the-art physical chemistry ideas and methods to the study of organic and inorganic compounds and macromolecules; polymeric, inorganic and composite materials as well as corresponding processes. The journal is intended for scientists in all fields of chemistry and in interdisciplinary sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


