Tunable acetylene sorption by flexible catenated metal–organic frameworks
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 25
Abstract
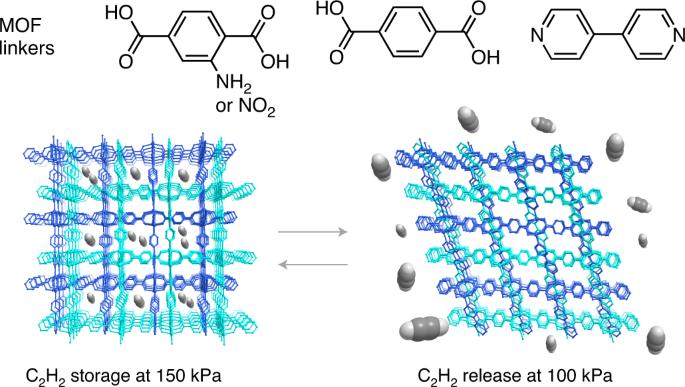
The safe storage of flammable gases, such as acetylene, is essential for current industrial purposes. However, the narrow pressure (P) and temperature range required for the industrial use of pure acetylene (100 < P < 200 kPa at 298 K) and its explosive behaviour at higher pressures make its storage and release challenging. Flexible metal–organic frameworks that exhibit a gated adsorption/desorption behaviour—in which guest uptake and release occur above threshold pressures, usually accompanied by framework deformations—have shown promise as storage adsorbents. Herein, the pressures for gas uptake and release of a series of zinc-based mixed-ligand catenated metal–organic frameworks were controlled by decorating its ligands with two different functional groups and changing their ratio. This affects the deformation energy of the framework, which in turn controls the gated behaviour. The materials offer good performances for acetylene storage with a usable capacity of ~90 v/v (77% of the overall amount) at 298 K and under a practical pressure range (100–150 kPa). Flexible metal–organic frameworks (MOFs) in which guest uptake and release occur above certain threshold pressures are attractive adsorbents. Now, the gated sorption behaviour of such a zinc-based mixed-ligand MOF has been tuned to match the narrow temperature and pressure range required for safe, efficient acetylene storage by adjusting the ratio of two different functional groups on its benzenedicarboxylate ligands.
柔性螯合金属有机框架的可调乙炔吸附
安全储存乙炔等易燃气体对当前的工业用途至关重要。然而,纯乙炔的工业用途所需的压力(P)和温度范围较窄(298 K 时为 100 < P < 200 kPa),而且在较高压力下具有爆炸性,这使得乙炔的储存和释放具有挑战性。表现出门控吸附/解吸行为的柔性金属有机框架--客体吸收和释放发生在阈值压力以上,通常伴随着框架变形--已显示出作为存储吸附剂的前景。在本文中,通过用两种不同的官能团装饰配体并改变它们的比例,控制了一系列锌基混合配体金属有机框架的气体吸收和释放压力。这会影响框架的变形能,进而控制门控行为。这些材料具有良好的乙炔储存性能,在 298 K 和实际压力范围(100-150 kPa)内的可用容量约为 90 v/v(占总量的 77%)。柔性金属有机框架(MOFs)是一种极具吸引力的吸附剂,在这种框架中,客体的吸收和释放均发生在一定的临界压力之上。现在,这种锌基混合配体 MOF 的门控吸附行为已经得到调整,通过调整其苯二甲酸配体上两种不同官能团的比例,使其符合安全、高效储存乙炔所需的狭窄温度和压力范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


