Remote C–H Functionalization of 8-Aminoquinoline Ring
IF 8.8
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 7
Abstract
8-Aminoquinoline is a common nitrogen-containing heterocyclic framework in many natural products, functional materials and useful drugs. It has been developed as a powerful bidentate directing group or ligand auxiliary in the field of C–H bond activation/functionalization in recent years. In this context, the synthesis of substituted 8-aminoquinoline is of great importance. In this review we focus on the functionalization of positions C2–C7 on the 8-aminoquinoline ring, which involves the formation of C–C and C–Z (Z?=?heteroatom) bonds by transition metal catalysts, photocatalysts or metal-free conditions. Mechanistically, a single electron transfer (SET) pathway is suggested in most cases.
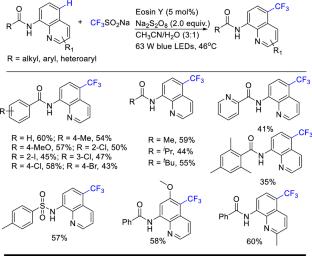
8-氨基喹啉环的远端碳氢功能化
8-氨基喹啉是许多天然产物、功能材料和有用药物中常见的含氮杂环骨架。近年来,它作为一种强有力的双齿导向基团或配体助剂在碳-氢键激活/功能化领域得到了发展。在此背景下,取代8-氨基喹啉的合成具有重要意义。本文综述了8-氨基喹啉环上C2-C7位的官能化,包括在过渡金属催化剂、光催化剂或无金属条件下形成C-C和C-Z (Z =?杂原子)键。在大多数情况下,单电子转移(SET)途径被认为是可行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Topics in Current Chemistry
Chemistry-General Chemistry
CiteScore
13.70
自引率
1.20%
发文量
48
期刊介绍:
Topics in Current Chemistry is a journal that presents critical reviews of present and future trends in modern chemical research. It covers all areas of chemical science, including interactions with related disciplines like biology, medicine, physics, and materials science. The articles in this journal are organized into thematic collections, offering a comprehensive perspective on emerging research to non-specialist readers in academia or industry. Each review article focuses on one aspect of the topic and provides a critical survey, placing it in the context of the collection. Selected examples highlight significant developments from the past 5 to 10 years. Instead of providing an exhaustive summary or extensive data, the articles concentrate on methodological thinking. This approach allows non-specialist readers to understand the information fully and presents the potential prospects for future developments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


