Enantioselective synthesis of tetraarylmethanes through meta-hydroxyl-directed benzylic substitution
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 2
Abstract
Benzylic carbocations bearing an ortho- or para-hydroxyl group can be stabilized by forming quinone methides, which have been explored in enantioselective synthesis. However, those with a meta-hydroxyl group have remained almost unexplored in organic synthesis. The lack of resonance stabilization by a typical quinone methide form renders them not only difficult to generate, but also challenging to control for asymmetric bond formation. Here we report an efficient catalytic enantioselective reaction between meta-hydroxyl triarylmethanols and indoles, via triaryl carbocations, for the synthesis of tetraarylmethanes with excellent enantiocontrol. Control experiments reveal that the meta-hydroxyl group is essential for both reactivity and stereocontrol. Ortho-directing groups (alkoxyl, sulfenyl or fluoro) benefit enantiocontrol through secondary hydrogen-bonding interactions, but are not required for reactivity. The resulting tetraarylmethane products show anticancer activities, through a mechanism distinct from that of classical anticancer drugs. The synthesis of benzylic carbocations bearing meta-hydroxyl substituents is difficult due to their lack of resonance stabilization. Now, a catalytic enantioselective reaction between meta-hydroxyl triarylmethanols and indoles, proceeding through a meta-hydroxyl triarylmethyl cation, is reported. A range of chiral tetraarylmethanes with anticancer activity are prepared.
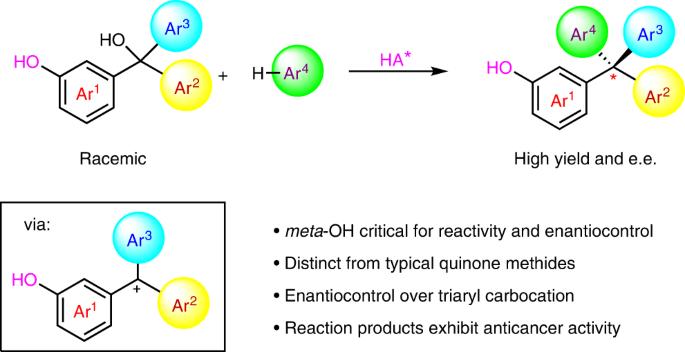
间羟基导向苯取代法对映选择性合成四芳基甲烷
带有正羟基或对羟基的羰基苄基化合物可以通过形成醌甲酰胺而得到稳定,这些醌甲酰胺已在对映选择性合成中进行了探索。然而,在有机合成中,那些带有元羟基的碳位体则几乎没有被探索过。典型的醌甲酰胺形式缺乏共振稳定,不仅难以生成,而且在控制不对称键的形成方面也具有挑战性。在此,我们报告了元羟基三芳基甲醇和吲哚之间通过三芳基碳化反应合成四芳基甲烷的高效催化对映体选择性反应,该反应具有极佳的对映体控制能力。对照实验表明,元羟基对反应活性和立体控制都至关重要。正向定向基团(烷氧基、亚磺酰基或氟基)通过二级氢键相互作用有利于对映体控制,但对反应性并非必需。由此产生的四芳基甲烷产品具有抗癌活性,其机理与传统抗癌药物不同。由于缺乏共振稳定性,很难合成带有元羟基取代基的苄基碳化物。现在,我们报道了一种通过偏羟基三芳基甲基阳离子在偏羟基三芳基甲醇和吲哚之间进行的催化对映体选择性反应。制备出了一系列具有抗癌活性的手性四芳基甲烷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


