Hydrogen bond-mediated organocatalytic enantioselective reduction of nitroalkenes in deep eutectic solvents
引用次数: 2
Abstract
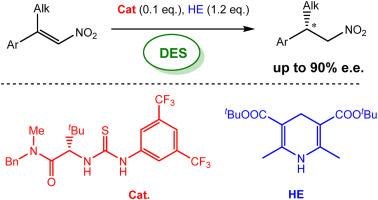
The catalytic enantioselective reduction of β,β-disubstituted nitroalkenes was performed in deep eutectic solvents, avoiding the use of volatile organic compounds (VOCs) as reaction medium. The desired enantioenriched nitroalkanes were obtained in high yields and high enantiomeric excesses, up to 90%, by a metal-free, hydrogen bond-mediated catalytic methodology, with a convenient experimental protocol that could be successfully applied to a gram-scale reaction.
深共晶溶剂中氢键介导的硝基烯烃的有机催化对映选择性还原
β,β-二取代硝基烯烃的催化对映选择性还原是在深共晶溶剂中进行的,避免了使用挥发性有机化合物(VOCs)作为反应介质。通过无金属、氢键介导的催化方法,获得了高产率和高对映体过量(高达90%)的富对映体硝基烷烃,并采用了方便的实验方案,可以成功地应用于克级反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


