Chiral control of spin-crossover dynamics in Fe(II) complexes
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 16
Abstract
Iron-based spin-crossover complexes hold tremendous promise as multifunctional switches in molecular devices. However, real-world technological applications require the excited high-spin state to be kinetically stable—a feature that has been achieved only at cryogenic temperatures. Here we demonstrate high-spin-state trapping by controlling the chiral configuration of the prototypical iron(II)tris(4,4′-dimethyl-2,2′-bipyridine) in solution, associated for stereocontrol with the enantiopure Δ- or Λ-enantiomer of tris(3,4,5,6-tetrachlorobenzene-1,2-diolato-κ2O1,O2)phosphorus(V) (P(O2C6Cl4)3– or TRISPHAT) anions. We characterize the high-spin-state relaxation using broadband ultrafast circular dichroism spectroscopy in the deep ultraviolet in combination with transient absorption and anisotropy measurements. We find that the high-spin-state decay is accompanied by ultrafast changes of its optical activity, reflecting the coupling to a symmetry-breaking torsional twisting mode, contrary to the commonly assumed picture. The diastereoselective ion pairing suppresses the vibrational population of the identified reaction coordinate, thereby achieving a fourfold increase of the high-spin-state lifetime. More generally, our results motivate the synthetic control of the torsional modes of iron(II) complexes as a complementary route to manipulate their spin-crossover dynamics. Despite much research, the high-spin-state relaxation mechanism of Fe(II) spin-crossover complexes is unresolved. Using ultrafast circular dichroism spectroscopy it has now been revealed that the spin relaxation is driven by a torsional twisting mode, which breaks the chiral symmetry of a prototypical Fe(II) compound. Stereocontrolling the configuration of the complex can thus be used to slow down the spin relaxation.
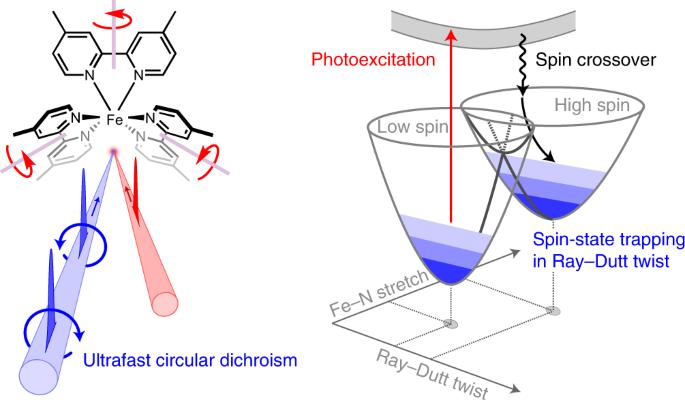
Fe(II)配合物自旋交叉动力学的手性控制
铁基自旋交叉复合物有望成为分子设备中的多功能开关。然而,现实世界中的技术应用需要激发的高自旋态具有动力学稳定性,而这一特性只有在低温条件下才能实现。在这里,我们通过控制原型铁(II)三(4,4′-二甲基-2,2′-联吡啶)在溶液中的手性构型,展示了高自旋态捕获、与三(3,4,5,6-四氯-1,2-苯二醇-κ2O1,O2)磷(V)(P(O2C6Cl4)3- 或 TRISPHAT)阴离子的对映体 Δ- 或Λ-对映体相关联,以实现立体控制。我们利用深紫外光下的宽带超快圆二色性光谱,结合瞬态吸收和各向异性测量,描述了高自旋态弛豫的特征。我们发现,高自旋态衰变伴随着其光学活性的超快变化,这反映了与对称性破坏扭转扭曲模式的耦合,这与通常假设的情况相反。非对映选择性离子配对抑制了已识别反应坐标的振动群,从而使高自旋态的寿命延长了四倍。更广泛地说,我们的研究结果推动了对铁(II)配合物的扭转模式进行合成控制,以此作为操纵其自旋交叉动力学的补充途径。尽管进行了大量研究,但铁(II)自旋交叉复合物的高自旋态弛豫机制仍未得到解决。通过使用超快圆二色性光谱,现在已经发现自旋弛豫是由扭转扭曲模式驱动的,这种模式打破了原型铁(II)化合物的手性对称性。因此,可以利用立体控制复合物的构型来减缓自旋弛豫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


