Synthesis, isolation and application of a sila-ketenyl anion
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
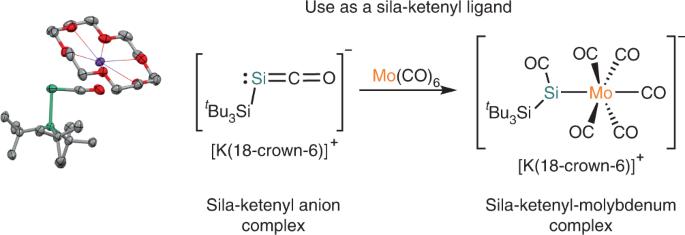
Ynolates or ketenyl anions, [RCCO]−, are negatively charged reactive intermediates, which can be generated in situ and used for divergent chemical transformations. Ynolates can react either at the oxygen or carbon centres or across the C–C triple bond, making them useful in various applications in organic synthesis. Heavier analogues of ynolates or ketenyl anions ([RECO]−, E = group 14 element), however, have not been isolated or studied. Here we report the synthesis, isolation and characterization of [K(18-crown-6)]+[(tBu3Si)SiCO]−, a silicon analogue of a ketenyl anion. [K(18-crown-6)]+[(tBu3Si)SiCO]− is readily prepared through reaction of [K(18-crown-6)]+ coordinated silyl-radical anions with carbon monoxide, or by a reduction of a silyl-substituted silicon–carbonyl complex, [{(Me3Si)3Si}(tBu3Si)SiCO]. X-ray crystallographic and spectroscopic analyses coupled with quantum chemical calculations reveal that [K(18-crown-6)]+[(tBu3Si)SiCO]− predominately displays sila-ketenyl anion character. [(tBu3Si)SiCO]− was also demonstrated to be a competent ligand for a transition metal through reaction with Mo(CO)6. The heavy analogues of ynolates or ketenyl anions have not yet been studied. Here the synthesis, isolation and characterization of a sila-ketenyl anion, the silicon analogue of a ketenyl anion, are reported through the reaction of silyl-radical anions with CO.
硅烯酮基阴离子的合成、分离及应用
乙醇酸盐或酮阴离子 [RCCO]- 是带负电荷的活性中间体,可就地生成并用于不同的化学转化。乙醇酸盐既可以在氧或碳中心发生反应,也可以跨越 C-C 三键发生反应,因此在有机合成的各种应用中非常有用。然而,炔醇酸盐或酮阴离子([RECO]-,E = 第 14 族元素)的较重类似物尚未被分离或研究。在此,我们报告了[K(18-crown-6)]+[(tBu3Si)SiCO]- 的合成、分离和表征,这是一种酮基阴离子的硅类似物。[K(18-crown-6)]+[(tBu3Si)SiCO]- 很容易通过[K(18-crown-6)]+配位硅基-自由基阴离子与一氧化碳的反应,或通过硅基取代硅-羰基复合物[{(Me3Si)3Si}(tBu3Si)SiCO]的还原反应制备。X 射线晶体学和光谱分析以及量子化学计算显示,[K(18-crown-6)]+[(tBu3Si)SiCO]- 主要具有硅酮阴离子的特征。通过与 Mo(CO)6 反应,[(tBu3Si)SiCO]- 还被证明是过渡金属的有效配体。人们尚未研究过乙醇酸盐或酮阴离子的重类似物。在此,我们报告了通过硅基自由基阴离子与 CO 的反应合成、分离和鉴定硅酮阴离子(酮阴离子的硅类似物)的情况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


