Forrestiacids C and D, unprecedented triterpene-diterpene adducts from Pseudotsuga forrestii
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 11
Abstract
Forrestiacids C (1) and D (2), a pair of C-25 epimeric triterpene–diterpene adducts were isolated from the needles and twigs of the vulnerable conifer Pseudotsuga forrestii. This unprecedented class of compounds might be generated via an intermolecular Michael addition reaction of a rearranged 6/6/5/5-fused spiro-lanostene with an abietene. Their structures were established by spectroscopic data and X-ray crystallography. The adducts showed inhibitory activities against the ATP-citrate lyase (ACL) and acetyl-CoA carboxylase 1 (ACC1), two rate-limiting enzymes in the de novo lipogenesis pathway.
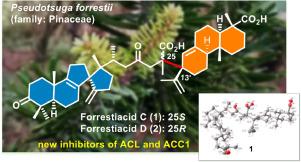
林分酸C和D,前所未有的三萜-二萜加合物
森林酸C(1)和D(2)是一对C-25表戊三萜-二萜加合物,从林分针叶和针叶中分离得到。这类前所未有的化合物可能是由重排的6/6/5/5-融合螺-烯与二烯的分子间Michael加成反应生成的。通过光谱数据和x射线晶体学确定了它们的结构。这些加合物对atp -柠檬酸裂解酶(ACL)和乙酰辅酶a羧化酶1 (ACC1)有抑制作用,这两种酶是新生脂肪生成途径中的限速酶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


