Photocatalytic Reduction of Co2 Under Visible Light with the Participation of Binary Cu2O/Ag2O Nanoheterostructures
IF 0.7
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Foam-like binary Cu2O/Ag2O nanoheterostructures have been obtained by an electrochemical method. Their photoelectrochemical and spectral properties have been studied. The photocatalytic properties of these composites in the process of gas-phase reduction of carbon dioxide with water vapor have been studied. The formation of binary nanoheterostructures leads to an increase in methane yield and an increase in the amount of other organic products (acetaldehyde, ethylene, and ethanol) while the system is irradiated with visible light when compared to Cu2O. This effect can be explained by the formation of a nanostructured Cu2O/Ag2O binary composite with a mutually consistent energy profile.
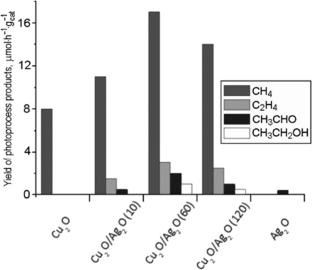
二元Cu2O/Ag2O纳米异质结构参与下可见光下Co2的光催化还原
采用电化学方法制备了泡沫状Cu2O/Ag2O二元纳米异质结构。研究了它们的光电化学和光谱性质。研究了这些复合材料在水蒸气气相还原二氧化碳过程中的光催化性能。与Cu2O相比,在可见光照射下,二元纳米异质结构的形成导致甲烷产量增加,其他有机产物(乙醛、乙烯和乙醇)的数量增加。这种效应可以通过形成具有相互一致能量分布的纳米结构Cu2O/Ag2O二元复合材料来解释。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


