B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 113
Abstract
B cells are a major component of the tumour microenvironment, where they are predominantly associated with tertiary lymphoid structures (TLS). In germinal centres within mature TLS, B cell clones are selectively activated and amplified, and undergo antibody class switching and somatic hypermutation. Subsequently, these B cell clones differentiate into plasma cells that can produce IgG or IgA antibodies targeting tumour-associated antigens. In tumours without mature TLS, B cells are either scarce or differentiate into regulatory cells that produce immunosuppressive cytokines. Indeed, different tumours vary considerably in their TLS and B cell content. Notably, tumours with mature TLS, a high density of B cells and plasma cells, as well as the presence of antibodies to tumour-associated antigens are typically associated with favourable clinical outcomes and responses to immunotherapy compared with those lacking these characteristics. However, polyclonal B cell activation can also result in the formation of immune complexes that trigger the production of pro-inflammatory cytokines by macrophages and neutrophils. In complement-rich tumours, IgG antibodies can also activate the complement cascade, resulting in the production of anaphylatoxins that sustain tumour-promoting inflammation and angiogenesis. Herein, we review the phenotypic heterogeneity of intratumoural B cells and the importance of TLS in their generation as well as the potential of B cells and TLS as prognostic and predictive biomarkers. We also discuss novel therapeutic approaches that are being explored with the aim of increasing mature TLS formation, B cell differentiation and anti-tumour antibody production within tumours. The tumour microenvironment includes various diverse immune cell types, each of which might influence tumour progression and response to treatment, particularly with immunotherapies. These cell types include different subtypes of B lymphocytes, which are often associated with tertiary lymphoid structures (TLS) and can have pro-tumour or anti-tumour effects, either through their classical function in antibody production and antigen presentation or other mechanisms. Herein, Fridman et al. discuss the phenotypic heterogeneity of intratumoural B cells and the importance of TLS in their generation, the potential of B cells and TLS as prognostic and/or predictive biomarkers, and novel approaches aiming to enhance the development of TLS and anti-tumour B cells for cancer therapy.
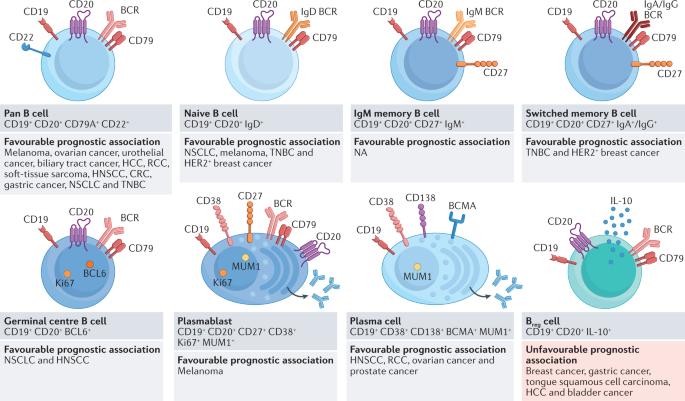
B细胞和三级淋巴结构作为肿瘤免疫环境和临床结果的决定因素
B 细胞是肿瘤微环境的主要组成部分,它们主要与三级淋巴结构(TLS)相关。在成熟的三级淋巴结构内的生殖中心,B 细胞克隆被选择性激活和扩增,并发生抗体类别转换和体细胞超突变。随后,这些 B 细胞克隆分化为浆细胞,可产生针对肿瘤相关抗原的 IgG 或 IgA 抗体。在没有成熟 TLS 的肿瘤中,B 细胞要么稀少,要么分化为产生免疫抑制细胞因子的调节细胞。事实上,不同肿瘤的 TLS 和 B 细胞含量差异很大。值得注意的是,与缺乏这些特征的肿瘤相比,具有成熟 TLS、高密度 B 细胞和浆细胞以及存在肿瘤相关抗原抗体的肿瘤通常具有良好的临床疗效和对免疫疗法的反应。然而,多克隆 B 细胞活化也会形成免疫复合物,引发巨噬细胞和中性粒细胞产生促炎细胞因子。在补体丰富的肿瘤中,IgG 抗体也能激活补体级联,导致产生苊毒素,维持肿瘤促炎症和血管生成。在此,我们回顾了肿瘤内 B 细胞的表型异质性和 TLS 在其生成过程中的重要性,以及 B 细胞和 TLS 作为预后和预测性生物标记物的潜力。我们还讨论了正在探索的新型治疗方法,这些方法旨在增加肿瘤内成熟 TLS 的形成、B 细胞分化和抗肿瘤抗体的产生。肿瘤微环境包括各种不同的免疫细胞类型,其中每一种都可能影响肿瘤的进展和对治疗的反应,尤其是对免疫疗法的反应。这些细胞类型包括不同亚型的B淋巴细胞,它们通常与三级淋巴结构(TLS)相关,可通过其产生抗体和抗原递呈的经典功能或其他机制产生促瘤或抗瘤作用。在本文中,Fridman 等人讨论了瘤内 B 细胞的表型异质性、三级淋巴结构在其生成过程中的重要性、B 细胞和三级淋巴结构作为预后和/或预测性生物标记物的潜力,以及旨在促进三级淋巴结构和抗肿瘤 B 细胞发展以治疗癌症的新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


