Lithiation–borylation methodology in the total synthesis of natural products
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 17
Abstract
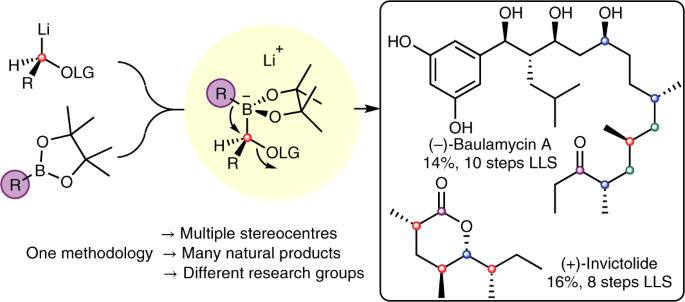
Robust synthetic methods that show a broad substrate scope are of great utility in the synthesis of complex organic molecules. Within this arena, synthetic methods that employ boronic esters are especially useful because they undergo a wide variety of transformations with very high levels of stereoselectivity. In particular, boronic esters can undergo single or multiple homologations using enantioenriched metal carbenoids. The addition of a suitable enantioenriched lithium or magnesium carbenoid to a boronic ester, with subsequent 1,2-migration, gives a homologated boronic ester with high stereocontrol. This process, termed lithiation–borylation, can be iterative, which allows a carbon chain to be extended one atom at a time with remarkable precision. The iterative homologation has been likened to a molecular assembly line and resembles the way nature assembles natural products, for example, in polyketide synthase machinery. The application of lithiation–borylation chemistry to the synthesis of a broad variety of natural products is discussed in this Review. Boronic esters are versatile intermediates that readily accept nucleophiles and then undergo 1,2-migration, expelling a neighbouring leaving group. Such reactivity enables carbon chains to be grown one atom at a time with high stereocontrol. This Review examines the fundamentals of lithiation–borylation methodology and its application to natural product synthesis.
天然产物全合成中的锂化-硼化方法
在合成复杂的有机分子时,具有广泛底物范围的可靠合成方法非常有用。在这一领域,采用硼酸酯的合成方法尤其有用,因为它们可以进行多种转化,并具有极高的立体选择性。特别是,硼酸酯可以使用对映体丰富的金属碳烯进行单一或多重同源转化。在硼酸酯中加入适当的对映体锂或镁类碳烯化合物,然后进行 1,2 迁移,就能得到具有高度立体控制的同系硼酸酯。这一过程被称为 "锂化-硼化"(lithiation-borylation),可以是迭代式的,这样就可以非常精确地一次延长一个原子的碳链。迭代同源化被比作分子组装线,类似于自然界组装天然产品的方式,例如在聚酮酸酯合成酶机器中。本综述讨论了锂化-硼化化学在合成多种天然产物中的应用。硼酸酯是用途广泛的中间体,容易接受亲核物,然后发生 1,2 迁移,排出邻近的离去基团。这种反应性使得碳链能够以高度的立体控制一次生长一个原子。本综述探讨了锂化-硼化方法的基本原理及其在天然产物合成中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


