Rotational spectroscopic studies of para-nitrobenzoic acid, para-aminobenzoic acid, para-chlorobenzoic acid, and para-hydroxybenzoic acid
Abstract
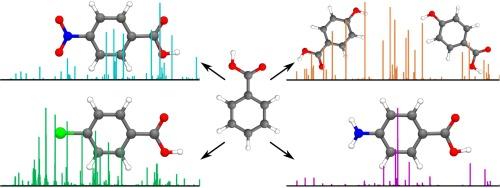
The rotational spectra of four substituted benzoic acids, i.e., para-aminobenzoic acid, para-nitrobenzoic acid, para-chlorobenzoic acid, and para-hydroxybenzoic acid were recorded with a chirp-pulse Fourier transform microwave spectrometer and analysed in terms of rotational constants and, where applicable, nuclear quadrupole coupling constants and tunnelling motions. In all instances, the experimentally identified conformer contains a carboxylic acid group in the cis-arrangement. In the case of para-hydroxybenzoic acid, spectra of two conformers were observed which differ in the orientation of the para-OH group. Quantum chemical calculations at the B3LYP-D3BJ/def2-TZVP and B2PLYP-D3BJ/cc-pCVTZ levels show that the trans-conformers are about 23 kJ mol−1 higher in energy than the global minimum cis-conformers. In general, the calculated rotational constants are in good agreement with the experimental derived ones. Agreement between calculated and experimental 14N and 35/37Cl nuclear quadrupole coupling constants is somewhat worse and necessitated the use of the B2PLYP functional in the case of para-nitrobenzoic acid to reduce the discrepancy. Conversion barriers between possible conformers were calculated to explain the absence of tunnelling splittings and/or higher energy conformers in the experimental spectra. An analysis of the electron density distribution was used to rationalize the calculated out-of-plane excursions of the carboxylic acid group in the trans-conformers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


