Entropy and sign conventions
IF 1.8
3区 化学
Q1 HISTORY & PHILOSOPHY OF SCIENCE
引用次数: 0
Abstract
It is a fundamental cornerstone of thermodynamics that entropy (\(S_{U,V}\)) increases in spontaneous processes in isolated systems (often called closed or thermally closed systems when the transfer of energy as work is considered to be negligible) and achieves a maximum when the system reaches equilibrium. But with a different sign convention entropy could just as well be said to decrease to a minimum in spontaneous constant U, V processes. It would then change in the same direction as the thermodynamic potentials in spontaneous processes. This article discusses but does not advocate such a change.
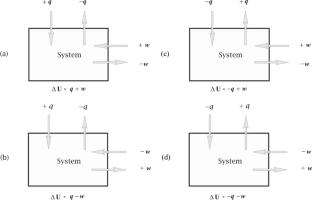
熵和符号约定
熵(\(S_{U,V}\))在孤立系统(通常称为封闭或热封闭系统,当能量作为功的传递被认为可以忽略不计时)的自发过程中增加,并在系统达到平衡时达到最大值,这是热力学的一个基本基石。但是用不同的符号约定,熵也可以说是在自发常数U, V过程中减小到最小值。它的变化方向与自发过程的热力学势相同。本文讨论但不提倡这种改变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Foundations of Chemistry
HISTORY & PHILOSOPHY OF SCIENCE-
自引率
22.20%
发文量
35
期刊介绍:
Foundations of Chemistry is an international journal which seeks to provide an interdisciplinary forum where chemists, biochemists, philosophers, historians, educators and sociologists with an interest in foundational issues can discuss conceptual and fundamental issues which relate to the `central science'' of chemistry. Such issues include the autonomous role of chemistry between physics and biology and the question of the reduction of chemistry to quantum mechanics. The journal will publish peer-reviewed academic articles on a wide range of subdisciplines, among others: chemical models, chemical language, metaphors, and theoretical terms; chemical evolution and artificial self-replication; industrial application, environmental concern, and the social and ethical aspects of chemistry''s professionalism; the nature of modeling and the role of instrumentation in chemistry; institutional studies and the nature of explanation in the chemical sciences; theoretical chemistry, molecular structure and chaos; the issue of realism; molecular biology, bio-inorganic chemistry; historical studies on ancient chemistry, medieval chemistry and alchemy; philosophical and historical articles; and material of a didactic nature relating to all topics in the chemical sciences. Foundations of Chemistry plans to feature special issues devoted to particular themes, and will contain book reviews and discussion notes. Audience: chemists, biochemists, philosophers, historians, chemical educators, sociologists, and other scientists with an interest in the foundational issues of science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


