High-entropy electrolytes for practical lithium metal batteries
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 3
Abstract
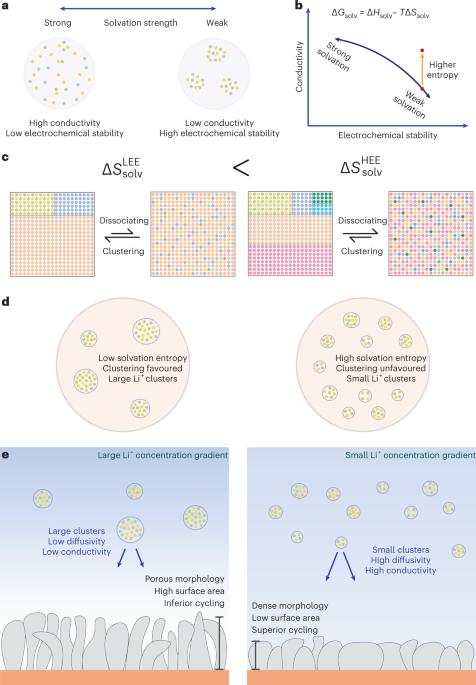
Electrolyte engineering is crucial for improving battery performance, particularly for lithium metal batteries. Recent advances in electrolytes have greatly improved cyclability by enhancing electrochemical stability at the electrode interfaces, but concurrently achieving high ionic conductivity has remained challenging. Here we report an electrolyte design strategy for enhanced lithium metal batteries by increasing the molecular diversity in electrolytes, which essentially leads to high-entropy electrolytes. We find that, in weakly solvating electrolytes, the entropy effect reduces ion clustering while preserving the characteristic anion-rich solvation structures, which is characterized by synchrotron-based X-ray scattering and molecular dynamics simulations. Electrolytes with smaller-sized clusters exhibit a twofold improvement in ionic conductivity compared with conventional weakly solvating electrolytes, enabling stable cycling at high current densities up to 2C (6.2 mA cm−2) in anode-free LiNi0.6Mn0.2Co0.2 (NMC622)||Cu pouch cells. The efficacy of the design strategy is verified by performance improvements in three disparate weakly solvating electrolyte systems. Electrolyte engineering has proven an effective approach to enhance the performance of lithium metal batteries. Here the authors propose a strategy by using multiple solvents in weakly solvating electrolytes—dubbed as high-entropy electrolytes—to improve the ionic conductivity while maintaining electrochemical stability, leading to high-performance batteries.
用于实用锂金属电池的高熵电解质
电解质工程对于提高电池性能至关重要,尤其是对于锂金属电池。电解质方面的最新进展通过提高电极界面的电化学稳定性,大大改善了循环性,但同时实现高离子电导率仍然具有挑战性。在此,我们报告了一种电解质设计策略,通过增加电解质中的分子多样性来提高锂金属电池的性能,这基本上导致了高熵电解质的产生。我们发现,在弱溶解电解质中,熵效应会减少离子团聚,同时保留富含阴离子的溶解结构特征,这可以通过基于同步辐射的 X 射线散射和分子动力学模拟来表征。与传统的弱溶解电解质相比,具有较小尺寸团簇的电解质的离子电导率提高了两倍,从而使无阳极镍钴锰酸锂(NMC622)||铜袋电池在高达 2C (6.2 mA cm-2)的高电流密度下实现了稳定循环。在三种不同的弱溶解电解质体系中的性能改善验证了设计策略的有效性。电解质工程已被证明是提高锂金属电池性能的有效方法。在此,作者提出了一种在弱溶解电解质(又称高熵电解质)中使用多种溶剂的策略,以在保持电化学稳定性的同时提高离子电导率,从而实现高性能电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


