Ternary ruthenium complex hydrides for ammonia synthesis via the associative mechanism
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 34
Abstract
Ammonia is the feedstock for nitrogen fertilizers and a potential carbon-free energy carrier; however, its production is highly energy intensive. Conventional heterogeneous catalysts based on metallic iron or ruthenium mediate dinitrogen dissociation and hydrogenation through a relatively energy-expensive pathway. Here we report the ternary ruthenium complex hydrides Li4RuH6 and Ba2RuH6 as an alternative class of catalysts, composed of electron- and hydrogen-rich [RuH6] anionic centres, for non-dissociative dinitrogen reduction, where hydridic hydrogen transports electrons and protons between the centres, and the Li/Ba cations stabilize NxHy (x = 0–2, y = 0–3) intermediates. The dynamic and synergistic involvement of all the components of the ternary complex hydrides facilitates an associative reaction mechanism with a narrow energy span and perfectly balanced kinetic barriers for the multistep process, leading to ammonia production from N2 + H2 with superior kinetics under mild conditions. The mechanism of ammonia synthesis on traditional iron or ruthenium catalysts features a high energetic span. Here, the authors introduce ternary ruthenium complex hydrides of lithium and barium that can activate dinitrogen via a lower-energy path, resulting in the highly efficient production of ammonia under milder conditions.
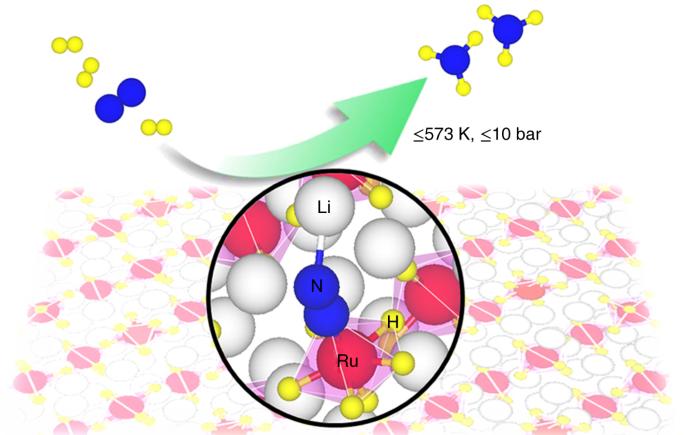
结合机理合成氨用三元钌络合氢化物
氨是氮肥的原料,也是一种潜在的无碳能源载体;然而,氨的生产需要大量能源。基于金属铁或钌的传统异相催化剂通过相对高能耗的途径介导二氮解离和氢化。在此,我们报告了三元钌络合物氢化物 Li4RuH6 和 Ba2RuH6,它们是由富含电子和氢的 [RuH6] 阴离子中心组成的另类催化剂,可用于非解离二氮还原,其中氢化物氢在中心之间传输电子和质子,而 Li/Ba 阳离子则稳定 NxHy(x = 0-2,y = 0-3)中间产物。三元复合氢化物中所有成分的动态协同参与,促进了多步过程中能量跨度小、动力学障碍完全平衡的关联反应机制,从而在温和条件下以优异的动力学性能从 N2 + H2 生成氨。在传统的铁或钌催化剂上合成氨的机理具有高能量跨度的特点。在这里,作者介绍了锂和钡的三元钌络合物氢化物,它们可以通过较低的能量途径激活二氮,从而在较温和的条件下高效生产氨。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


