Chemotherapy-induced loss of bone and muscle mass in a mouse model of breast cancer bone metastases and cachexia
Abstract
Background
Chemotherapy used to treat malignancy can lead to loss of skeletal muscle mass and reduced force production, and can reduce bone volume in mice. We have shown that bone-muscle crosstalk is a key nexus in skeletal muscle function and bone homeostasis in osteolytic breast cancer bone metastases. Because chemotherapy has significant negative side effects on bone mass, and because bone loss can drive skeletal muscle weakness, we have examined the effects of chemotherapy on the musculoskeletal system in mice with breast cancer bone metastases.
Methods and results
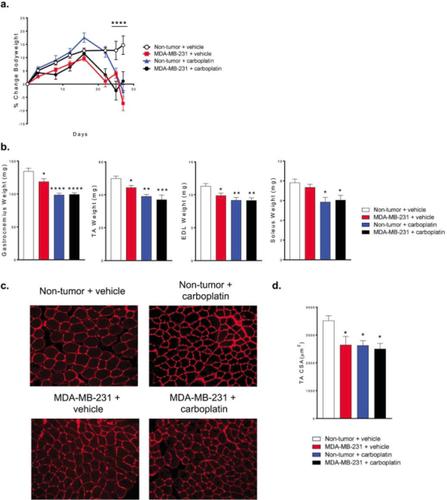
Six-week-old Female athymic nude mice were inoculated with 105 MDA-MB231 human breast cancer cells into the left ventricle and bone metastases were confirmed by X-ray. Mice were injected with carboplatin at a dose of 60mg/kg once per week starting 4 days after tumor inoculation. Skeletal muscle was collected for biochemical analysis and extensor digitorum longus (EDL) whole muscle contractility was measured. The femur and tibia bone parameters were assessed by microCT and tumor burden in bone was determined by histology. Healthy mice treated with carboplatin lose whole body weight and have reduced individual muscle weights (gastrocnemius, tibialis anterior (TA), and EDL), reduced trabecular bone volume (BV/TV), and reduced EDL function. Mice with MDA-MB-231 bone metastases treated with carboplatin lose body weight, and have reduced EDL function as healthy mice treated with carboplatin. Mice with MDA-MB-231 bone metastases plus carboplatin do have reduced proximal tibia BV/TV compared to carboplatin alone, but carboplatin does reduce tumor burden in bone.
Conclusions
Our data shows that carboplatin treatment, aimed at reducing tumor burden, contributes to cachexia and trabecular bone loss. The muscle atrophy and weakness may occur through bone-muscle crosstalk and would lead to a feed-forward cycle of musculoskeletal degradation. Despite anti-tumor effects of chemotherapy, musculoskeletal impairment is still significant in mice with bone metastases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


