Michael High, Qilei Song, Kyra L. Sedransk Campbell, Paul S. Fennell
下载PDF
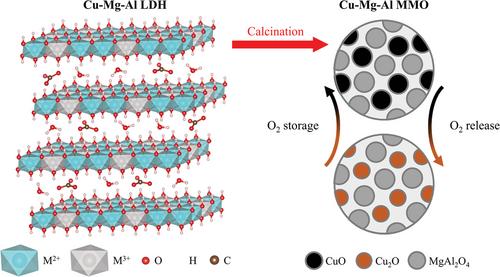
{"title":"Layered double hydroxide-derived copper-based oxygen carriers for chemical looping applications: Oxygen release kinetics and impact of loading on long-term performance","authors":"Michael High, Qilei Song, Kyra L. Sedransk Campbell, Paul S. Fennell","doi":"10.1002/ghg.2214","DOIUrl":null,"url":null,"abstract":"<p>Chemical looping with oxygen uncoupling, a variant of chemical looping combustion, requires chemically and physically stable oxygen carriers over long-term redox cycling. Copper-based oxygen carriers are characterised by high oxygen release rates but experience sintering at high temperatures. The use of layered double hydroxides (LDHs), prepared <i>via</i> co-precipitation, as oxygen carrier precursors has been shown to effectively limit deactivation of copper-based mixed metal oxides (MMOs) over extended redox cycling. The LDH-derived MMOs have highly dispersed metal oxide within a stable support; the high dispersion of metals is due to the LDH precursor structure. In this work, a fluidised bed reactor (FBR) was used to study the intrinsic kinetics of oxygen release from CuO/MgAl<sub>2</sub>O<sub>4</sub> oxygen carriers synthesised <i>via</i> the LDH-MMO design strategy. The long-term performance of MMOs with higher loadings of CuO, calcined from LDHs with higher Cu contents, was also investigated using an FBR. The intrinsic kinetics were determined using a kinetic model incorporating an effectiveness factor. By minimising the effects of intra- and inter-particle mass transfer, the activation energy and the pre-exponential factor of the lower loading MMOs were determined to be 51 ± 3 kJ mol<sup>−1</sup> and 0.0567 s<sup>−1</sup>, respectively. All MMOs showed excellent stability over 100 redox cycles in a thermogravimetric analyser. However, the pH during co-precipitation of the LDHs affected the stability of the MMOs in an FBR. The MMOs calcined from LDHs synthesised at pH 9.5 disintegrated during operation, whilst those produced from LDHs synthesised at pH 11 maintained high conversion and physical integrity over 100 redox cycles. © 2023 The Authors. <i>Greenhouse Gases: Science and Technology</i> published by Society of Chemical Industry and John Wiley & Sons Ltd.</p>","PeriodicalId":12796,"journal":{"name":"Greenhouse Gases: Science and Technology","volume":"13 4","pages":"535-545"},"PeriodicalIF":2.7000,"publicationDate":"2023-03-27","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/ghg.2214","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Greenhouse Gases: Science and Technology","FirstCategoryId":"93","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/ghg.2214","RegionNum":4,"RegionCategory":"环境科学与生态学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q3","JCRName":"ENERGY & FUELS","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Chemical looping with oxygen uncoupling, a variant of chemical looping combustion, requires chemically and physically stable oxygen carriers over long-term redox cycling. Copper-based oxygen carriers are characterised by high oxygen release rates but experience sintering at high temperatures. The use of layered double hydroxides (LDHs), prepared via co-precipitation, as oxygen carrier precursors has been shown to effectively limit deactivation of copper-based mixed metal oxides (MMOs) over extended redox cycling. The LDH-derived MMOs have highly dispersed metal oxide within a stable support; the high dispersion of metals is due to the LDH precursor structure. In this work, a fluidised bed reactor (FBR) was used to study the intrinsic kinetics of oxygen release from CuO/MgAl2 O4 oxygen carriers synthesised via the LDH-MMO design strategy. The long-term performance of MMOs with higher loadings of CuO, calcined from LDHs with higher Cu contents, was also investigated using an FBR. The intrinsic kinetics were determined using a kinetic model incorporating an effectiveness factor. By minimising the effects of intra- and inter-particle mass transfer, the activation energy and the pre-exponential factor of the lower loading MMOs were determined to be 51 ± 3 kJ mol−1 and 0.0567 s−1 , respectively. All MMOs showed excellent stability over 100 redox cycles in a thermogravimetric analyser. However, the pH during co-precipitation of the LDHs affected the stability of the MMOs in an FBR. The MMOs calcined from LDHs synthesised at pH 9.5 disintegrated during operation, whilst those produced from LDHs synthesised at pH 11 maintained high conversion and physical integrity over 100 redox cycles. © 2023 The Authors. Greenhouse Gases: Science and Technology published by Society of Chemical Industry and John Wiley & Sons Ltd.
化学环应用的层状双氢氧衍生铜基氧载体:氧释放动力学和负载对长期性能的影响
氧解偶联化学环是化学环燃烧的一种变体,需要长期氧化还原循环中化学和物理上稳定的氧载体。铜基氧载体的特点是氧释放速率高,但在高温下会发生烧结。通过共沉淀法制备的层状双氢氧化物(LDHs)作为氧载体前体已被证明可以有效地限制铜基混合金属氧化物(MMOs)在延长氧化还原循环中的失活。ldh衍生的mmo在稳定的载体内具有高度分散的金属氧化物;金属的高分散是由于LDH前驱体的结构。本文采用流化床反应器(FBR)研究了采用LDH-MMO设计策略合成的CuO/MgAl2O4氧载体的氧释放动力学特性。用FBR研究了高Cu含量的LDHs煅烧高CuO负载的MMOs的长期性能。本征动力学是用动力学模型确定了一个有效因素。通过最小化粒子内和粒子间传质的影响,确定了低负荷MMOs的活化能和指前因子分别为51±3 kJ mol−1和0.0567 s−1。所有MMOs在热重分析仪中表现出超过100次氧化还原循环的优异稳定性。然而,LDHs共沉淀过程中的pH值影响了FBR中MMOs的稳定性。在pH 9.5下合成的LDHs煅烧出的MMOs在运行过程中分解,而在pH 11下合成的LDHs产生的MMOs在100次氧化还原循环中保持了高转化率和物理完整性。©2023作者。温室气体:科学与技术,化学工业学会和约翰·威利出版社出版;子有限公司
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


