A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 140
Abstract
Current electrophysiological or optical techniques cannot reliably perform simultaneous intracellular recordings from more than a few tens of neurons. Here we report a nanoelectrode array that can simultaneously obtain intracellular recordings from thousands of connected mammalian neurons in vitro. The array consists of 4,096 platinum-black electrodes with nanoscale roughness fabricated on top of a silicon chip that monolithically integrates 4,096 microscale amplifiers, configurable into pseudocurrent-clamp mode (for concurrent current injection and voltage recording) or into pseudovoltage-clamp mode (for concurrent voltage application and current recording). We used the array in pseudovoltage-clamp mode to measure the effects of drugs on ion-channel currents. In pseudocurrent-clamp mode, the array intracellularly recorded action potentials and postsynaptic potentials from thousands of neurons. In addition, we mapped over 300 excitatory and inhibitory synaptic connections from more than 1,700 neurons that were intracellularly recorded for 19 min. This high-throughput intracellular-recording technology could benefit functional connectome mapping, electrophysiological screening and other functional interrogations of neuronal networks. An electronic interface with 4,096 electrodes can intracellularly record postsynaptic potentials and action potentials from thousands of connected mammalian neurons in vitro.
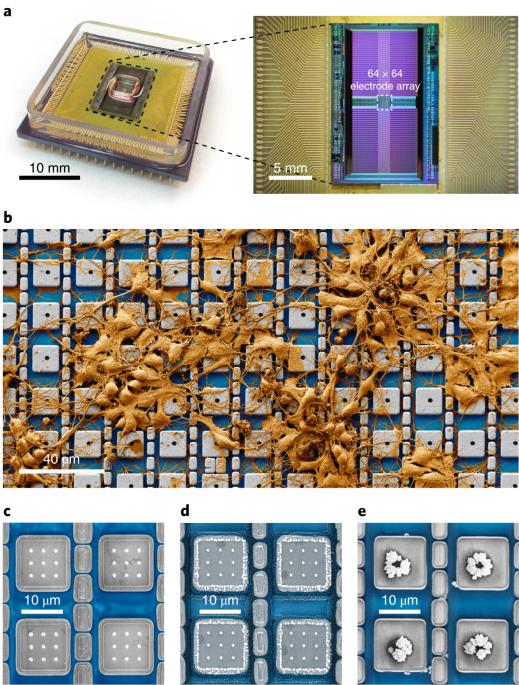
一种用于从数千个连接的神经元获得细胞内记录的纳米电极阵列
目前的电生理或光学技术无法可靠地同时对几十个以上的神经元进行胞内记录。在这里,我们报告了一种纳米电极阵列,它能同时获得数千个体外连接的哺乳动物神经元的胞内记录。该阵列由 4096 个具有纳米级粗糙度的黑铂电极组成,这些电极位于硅芯片之上,硅芯片单片集成了 4096 个微型放大器,可配置为伪电流钳模式(用于同时注入电流和记录电压)或伪电压钳模式(用于同时施加电压和记录电流)。我们在假电压钳模式下使用该阵列测量药物对离子通道电流的影响。在伪电流钳模式下,阵列在细胞内记录了数千个神经元的动作电位和突触后电位。此外,我们还绘制了来自 1700 多个神经元的 300 多个兴奋性和抑制性突触连接图,这些神经元在细胞内记录了 19 分钟。这种高通量的细胞内记录技术将有利于功能性连接组图谱绘制、电生理筛选和神经元网络的其他功能性研究。一个带有4,096个电极的电子接口可以在体外对数千个相连的哺乳动物神经元的突触后电位和动作电位进行细胞内记录。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


