Nucleophilic Aromatic Substitution of 5-Bromo-1,2,3-triazines with Phenols
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
Nucleophilic aromatic substitution (SNAr) reaction in classic textbook is a stepwise mechanism, and few examples of concerted reactions have been reported. Herein, we developed a concerted SNAr reaction of 5-bromo-1,2,3-triazines with phenols in which the nonclassic mechanism of this reaction could be revealed by calculation. Furthermore, the resulting 5-aryloxy-1,2,3-triazines could be used as convenient precursors to access biologically important 3-aryloxy-pyridines in one-pot manner.
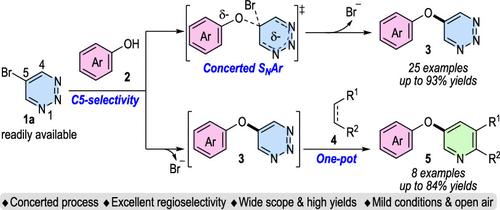
苯酚取代5-溴-1,2,3-三嗪的亲核芳香性
亲核芳烃取代反应(SNAr)在经典教科书中是一个阶梯反应机制,很少有协调反应的例子报道。本文建立了5-溴-1,2,3-三嗪类化合物与酚类化合物的协同SNAr反应,并通过计算揭示了该反应的非经典机理。此外,所得的5-芳氧基-1,2,3-三嗪可以作为方便的前体,以一锅法获得具有重要生物学意义的3-芳氧基吡啶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


