Photochemical Synthesis of Pyrazolines from Tetrazoles in Flow
IF 2.1
Q2 CHEMISTRY, ORGANIC
引用次数: 1
Abstract
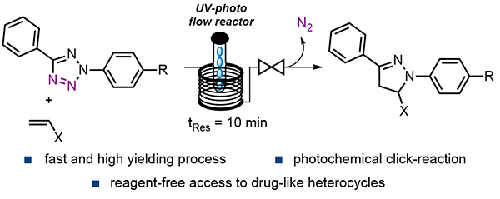
Pyrazolines and their pyrazole congeners are important heterocyclic building blocks with numerous applications in the fine chemical industries. However, traditional routes towards these entities are based on multistep syntheses generating substantial amounts of chemical waste. Here we report an alternative approach using UV-light to convert tetrazoles to pyrazolines via a reagent-free photo-click strategy. This route generates nitrile imine dipoles in situ that are trapped with different dipolarophiles rendering a selection of these heterocyclic targets in high chemical yields. A continuous flow method is ultimately realized that generates multi-gram quantities of product in a safe and readily scalable manner thus demonstrating the value of this photochemical approach for future exploitations in industry.
四氮唑光化学合成吡唑啉的研究
吡唑啉及其吡唑同系物是重要的杂环化合物,在精细化工中有着广泛的应用。然而,通往这些实体的传统路线是基于产生大量化学废物的多步骤合成。在这里,我们报告了一种替代方法,利用紫外线通过无试剂的照片点击策略将四唑转化为吡唑啉。该方法在原位产生腈亚胺偶极子,这些偶极子被不同的亲偶极试剂捕获,从而在高化学收率中选择这些杂环目标。最终实现了一种连续流动方法,以安全且易于扩展的方式生成数克数量的产品,从而证明了这种光化学方法在未来工业开发中的价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


