Protecting Group-Free Gold-Catalyzed Synthesis of 2-Acylidene-3-Oxindoles and Azaaurones via a Double Oxidation Strategy
IF 2.3
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A one-pot synthesis of 2-acylidene-3-oxindole and azaaurone derivatives starting from O-alkynylanilines and alkynes is presented. By means of oxidative gold catalysis the two starting materials are transferred to reactive intermediates that in situ form the target products. This double oxidation strategy enables a protecting group-free step-economic strategy towards these valuable substrate classes.
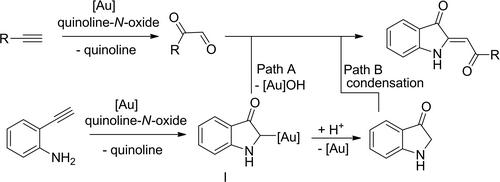
通过双氧化策略保护无基金催化合成2 -酰基- 3 -氧吲哚和氮唑酮
介绍了以O -炔基苯胺和炔为起始原料,一锅法合成2 -酰基- 3 -氧吲哚和氮唑酮衍生物的方法。通过氧化金催化,将两种起始材料转移到原位形成目标产物的反应中间体。这种双重氧化策略使得对这些有价值的基材类别的保护组-无步骤-经济策略成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Israel Journal of Chemistry
化学-化学综合
CiteScore
6.20
自引率
0.00%
发文量
62
审稿时长
6-12 weeks
期刊介绍:
The fledgling State of Israel began to publish its scientific activity in 1951 under the general heading of Bulletin of the Research Council of Israel, which quickly split into sections to accommodate various fields in the growing academic community. In 1963, the Bulletin ceased publication and independent journals were born, with Section A becoming the new Israel Journal of Chemistry.
The Israel Journal of Chemistry is the official journal of the Israel Chemical Society. Effective from Volume 50 (2010) it is published by Wiley-VCH.
The Israel Journal of Chemistry is an international and peer-reviewed publication forum for Special Issues on timely research topics in all fields of chemistry: from biochemistry through organic and inorganic chemistry to polymer, physical and theoretical chemistry, including all interdisciplinary topics. Each topical issue is edited by one or several Guest Editors and primarily contains invited Review articles. Communications and Full Papers may be published occasionally, if they fit with the quality standards of the journal. The publication language is English and the journal is published twelve times a year.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


