High-purity electrolytic lithium obtained from low-purity sources using solid electrolyte
IF 27.1
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 39
Abstract
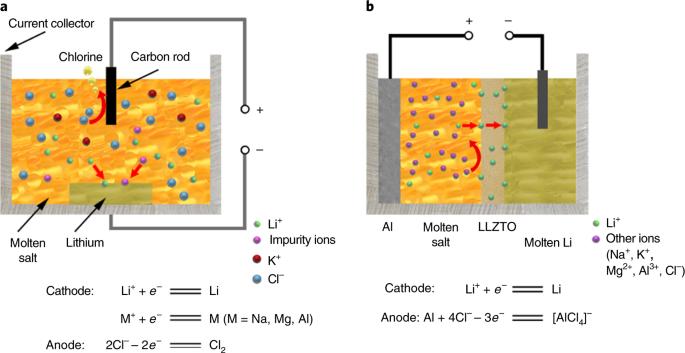
Lithium (Li) is an important resource for the sustainability of socioeconomic systems given its wide use in various industrial applications. The industrial production of Li metals relies on the electrolysis of a mixture consisting of high-purity lithium chloride (LiCl) and potassium chloride. However, the purification of LiCl is expensive and unsustainable, requiring a substantial amount of energy and the use of noxious chemical reagents, so that producing high-purity Li efficiently and sustainably is a challenge. Herein we report a new method of producing high-purity electrolytic Li from low-purity LiCl using solid-state electrolyte. Taking advantage of the high Li-ion selectivity of the solid electrolyte, we directly obtained high-purity metallic Li through the electrolysis of low-purity LiCl. Our new method provides two important advantages over conventional methods: (1) the cost of producing high-purity Li is reduced by using low-purity LiCl from low-grade brine, and the simpler purification process reduces the use of energy and chemical reagents; and (2) the operating temperature of the electrolytic process decreases from 400 °C to 240 °C, leading to an additional reduction in energy use. The production of lithium requires the purification of lithium chloride, which is expensive and unsustainable. A new method allows the production of high-purity electrolytic lithium from low-purity lithium chloride using solid-state electrolyte, with substantial reductions in costs and environmental impacts.
用固体电解质从低纯度来源获得的高纯度电解锂
鉴于锂(Li)在各种工业应用中的广泛使用,它是社会经济系统可持续发展的重要资源。锂金属的工业生产依赖于电解由高纯度氯化锂(LiCl)和氯化钾组成的混合物。然而,氯化锂的提纯过程既昂贵又不可持续,需要消耗大量能源并使用有毒化学试剂,因此高效、可持续地生产高纯度锂是一项挑战。在此,我们报告了一种利用固态电解质从低纯氯化锂生产高纯电解锂的新方法。利用固态电解质的高锂离子选择性,我们通过电解低纯氯化锂直接获得了高纯金属锂。与传统方法相比,我们的新方法有两个重要优势:(1) 从低品位盐水中提取低纯氯化锂,降低了生产高纯锂的成本,而且纯化过程更简单,减少了能源和化学试剂的使用;(2) 电解过程的操作温度从 400 ℃ 降至 240 ℃,从而进一步减少了能源使用。锂的生产需要对氯化锂进行提纯,这既昂贵又不可持续。一种新方法可以利用固态电解质从低纯度氯化锂中生产出高纯度电解锂,从而大幅降低成本并减少对环境的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Sustainability
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
41.90
自引率
1.10%
发文量
159
期刊介绍:
Nature Sustainability aims to facilitate cross-disciplinary dialogues and bring together research fields that contribute to understanding how we organize our lives in a finite world and the impacts of our actions.
Nature Sustainability will not only publish fundamental research but also significant investigations into policies and solutions for ensuring human well-being now and in the future.Its ultimate goal is to address the greatest challenges of our time.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


