Highly Efficient Synthesis of 3,3-Disubstituted Oxindoles through Direct Oxidative Alkylarylation of N -Arylacrylamides with Simple Alkanes
IF 2.1
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A direct oxidative alkylarylation reaction of N-arylacrylamides with simple alkanes for the synthesis of 3,3-disubstituted oxindoles under metal-free conditions was demonstrated. By using PhI(OAc)2 [(diacetoxy)iodobenzene] as an oxidant, a series of 3,3-disubstituted oxindoles bearing different aryl or alkyl substituents were generated in moderate to excellent chemical yields via a radical-initiated alkylation/cyclization process. The reported method features good functional group tolerance and wide substrate range, and provides an effective method for the preparation of various alkyl substituted 3,3-disubstituted oxindoles.
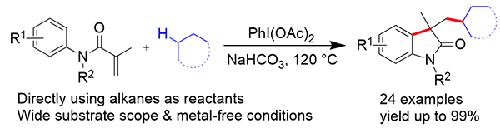
N -芳烯酰胺与简单烷烃直接氧化烷基化高效合成3,3-二取代氧吲哚
研究了n -芳基丙烯酰胺与简单烷烃在无金属条件下直接氧化烷基化合成3,3-二取代氧吲哚的反应。以PhI(OAc)2[(二乙酰氧基)碘苯]为氧化剂,通过自由基引发的烷基化/环化反应,生成了一系列含有不同芳基或烷基取代基的3,3-二取代氧吲哚,收率中等至优异。该方法具有官能团耐受性好、底物范围广的特点,为制备各种烷基取代3,3-二取代氧吲哚提供了一种有效的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


