Rhodium-catalyzed asymmetric hydroamination of gem-difluoroallenes with anilines
引用次数: 0
Abstract
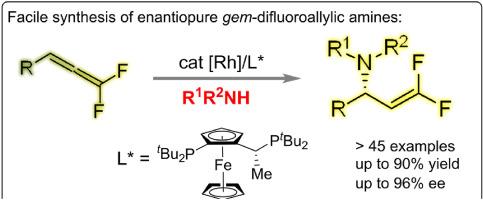
A rhodium-catalyzed protocol has been established for the enantioselective hydroamination of gem-difluoroallenes with anilines. This strategy shows complete atom-efficiency to access a variety of enantioenriched gem-difluoroallylic amines in excellent branched selectivity (>99:1 for each case), which are important backbones in many biologically active compounds. Mechanistic studies suggest the generation of a gem-difluoro π-allyl rhodium intermediate by an exclusive hydrometalation pathway, which undergoes nucleophilic substitution of amines.
铑催化的宝石二氟烯与苯胺的不对称氢胺化反应
建立了一种铑催化的宝石二氟烯与苯胺的对映选择性氢胺化反应方案。这一策略显示了完全的原子效率,以极好的支链选择性(>99:1)获得各种对映体富集的宝石-二氟烯丙胺,这是许多生物活性化合物的重要骨干。机理研究表明,宝石-二氟π-烯丙基铑中间体的生成是通过纯金属加氢化途径,经过胺的亲核取代。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


