Striatal Dopamine Signals and Reward Learning.
IF 3.8
Q2 CELL BIOLOGY
Function (Oxford, England)
Pub Date : 2023-10-03
eCollection Date: 2023-01-01
DOI:10.1093/function/zqad056
引用次数: 0
Abstract
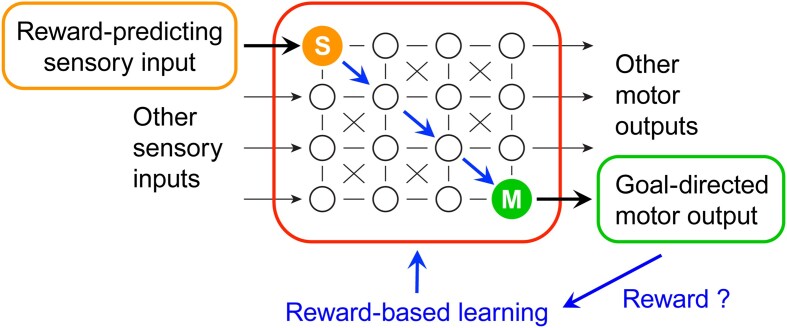
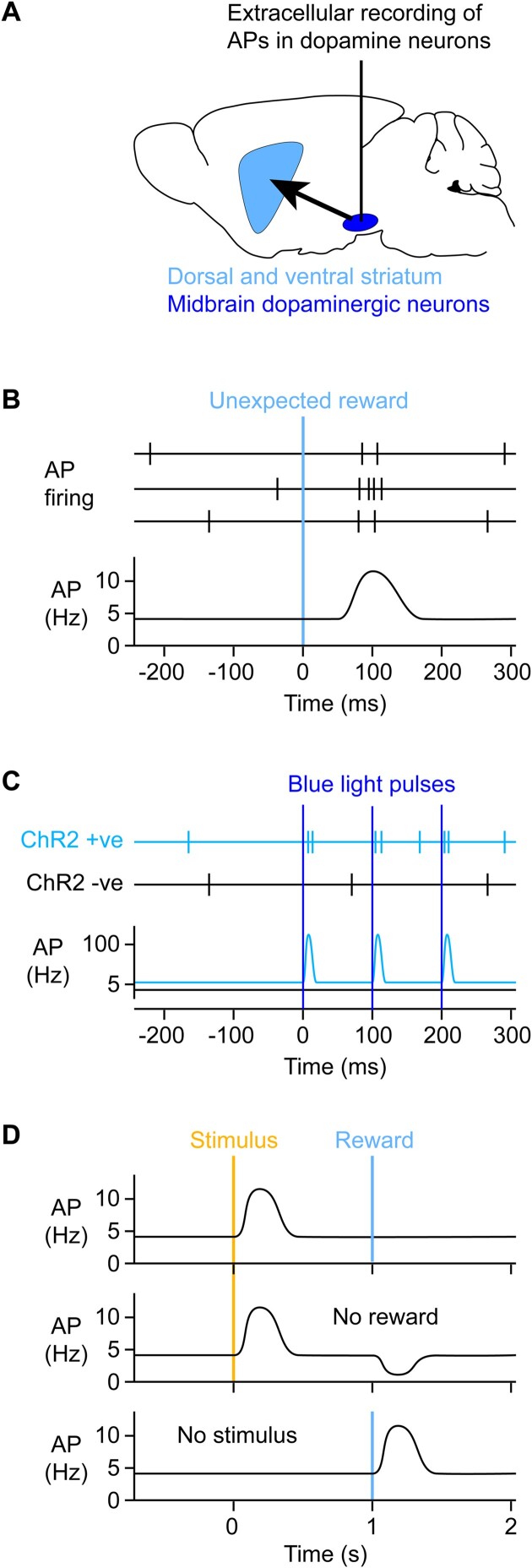
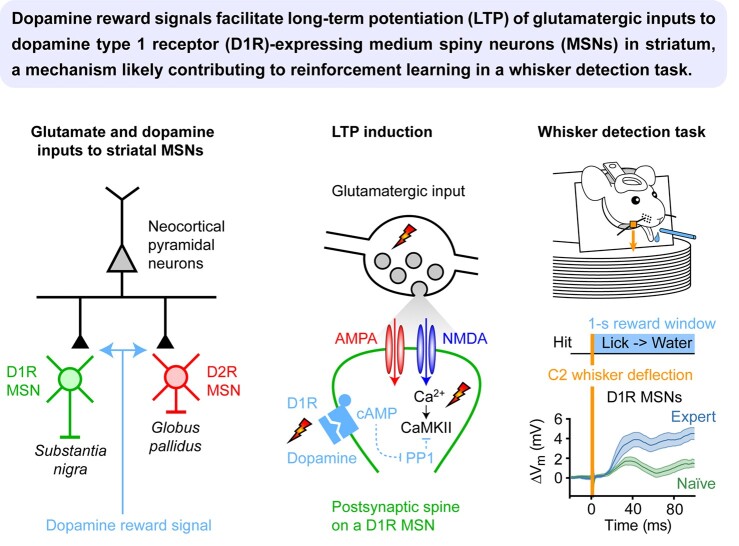
Abstract We are constantly bombarded by sensory information and constantly making decisions on how to act. In order to optimally adapt behavior, we must judge which sequences of sensory inputs and actions lead to successful outcomes in specific circumstances. Neuronal circuits of the basal ganglia have been strongly implicated in action selection, as well as the learning and execution of goal-directed behaviors, with accumulating evidence supporting the hypothesis that midbrain dopamine neurons might encode a reward signal useful for learning. Here, we review evidence suggesting that midbrain dopaminergic neurons signal reward prediction error, driving synaptic plasticity in the striatum underlying learning. We focus on phasic increases in action potential firing of midbrain dopamine neurons in response to unexpected rewards. These dopamine neurons prominently innervate the dorsal and ventral striatum. In the striatum, the released dopamine binds to dopamine receptors, where it regulates the plasticity of glutamatergic synapses. The increase of striatal dopamine accompanying an unexpected reward activates dopamine type 1 receptors (D1Rs) initiating a signaling cascade that promotes long-term potentiation of recently active glutamatergic input onto striatonigral neurons. Sensorimotor-evoked glutamatergic input, which is active immediately before reward delivery will thus be strengthened onto neurons in the striatum expressing D1Rs. In turn, these neurons cause disinhibition of brainstem motor centers and disinhibition of the motor thalamus, thus promoting motor output to reinforce rewarded stimulus-action outcomes. Although many details of the hypothesis need further investigation, altogether, it seems likely that dopamine signals in the striatum might underlie important aspects of goal-directed reward-based learning.
纹状体多巴胺信号与奖励学习。
我们不断地被感官信息轰炸,并不断地做出如何行动的决定。为了最佳地适应行为,我们必须判断在特定情况下,哪些感官输入和动作序列会带来成功的结果。基底神经节的神经元回路与动作选择以及目标导向行为的学习和执行密切相关,越来越多的证据支持中脑多巴胺神经元可能编码对学习有用的奖励信号的假设。在这里,我们回顾了一些证据,这些证据表明中脑多巴胺能神经元发出奖励预测错误的信号,驱动学习背后纹状体的突触可塑性。我们关注中脑多巴胺神经元对意外奖励的动作电位放电的阶段性增加。这些多巴胺神经元显著支配背侧和腹侧纹状体。在纹状体中,释放的多巴胺与多巴胺受体结合,在那里调节谷氨酸能突触的可塑性。伴随着意外奖励的纹状体多巴胺的增加激活了多巴胺1型受体(D1Rs),启动了一个信号级联,促进了最近活跃的谷氨酸能输入对纹状体神经元的长期增强。感觉运动诱发的谷氨酸能输入,在奖赏传递之前立即活跃,因此将增强到纹状体中表达D1Rs的神经元上。反过来,这些神经元引起脑干运动中心的去抑制和运动丘脑的去抑制,从而促进运动输出,以加强奖励刺激动作的结果。尽管该假说的许多细节需要进一步研究,但纹状体中的多巴胺信号似乎可能是目标导向的基于奖励的学习的重要方面的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


