Adjuvant nivolumab in resected stage IIB/C melanoma: primary results from the randomized, phase 3 CheckMate 76K trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 2
Abstract
Patients with resected stage IIB/C melanoma have high recurrence risk, similar to those with resected stage IIIA/B disease. The phase 3, double-blind CheckMate 76K trial assessed 790 patients with resected stage IIB/C melanoma randomized 2:1 (stratified by tumor category) to nivolumab 480 mg or placebo every 4 weeks for 12 months. The primary endpoint was investigator-assessed recurrence-free survival (RFS). Secondary endpoints included distant metastasis-free survival (DMFS) and safety. At 7.8 months of minimum follow-up, nivolumab significantly improved RFS versus placebo (hazard ratio (HR) = 0.42; 95% confidence interval (CI): 0.30–0.59; P < 0.0001), with 12-month RFS of 89.0% versus 79.4% and benefit observed across subgroups; DMFS was also improved (HR = 0.47; 95% CI: 0.30–0.72). Treatment-related grade 3/4 adverse events occurred in 10.3% (nivolumab) and 2.3% (placebo) of patients. One treatment-related death (0.2%) occurred with nivolumab. Nivolumab is an effective and generally well-tolerated adjuvant treatment in patients with resected stage IIB/C melanoma. ClinicalTrials.gov identifier: NCT04099251 . In this pre-specified interim analysis, patients with resected stage IIB/C melanoma who received adjuvant nivolumab had significantly prolonged recurrence-free survival compared to placebo-treated patients, providing another treatment option for this population.
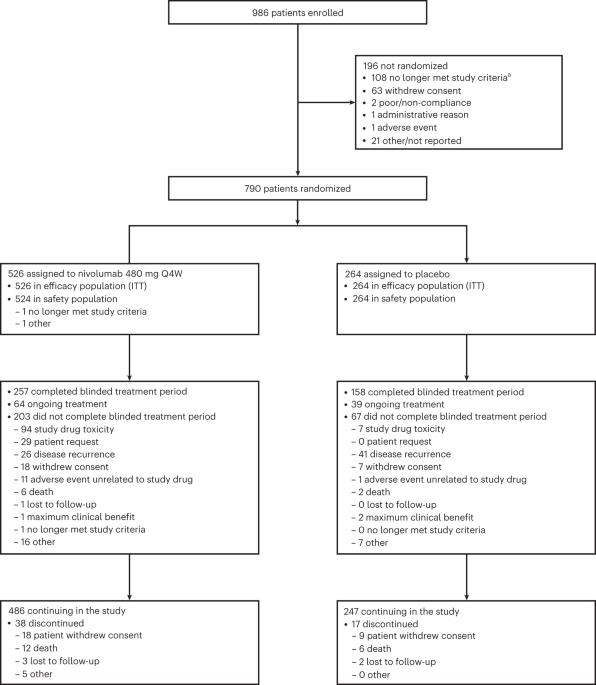
nivolumab佐剂治疗切除的IIB/C期黑色素瘤:CheckMate 76K随机3期试验的主要结果。
切除的IIB/C期黑色素瘤患者有很高的复发风险,与切除的IIIA/B期疾病患者相似。CheckMate 76K的3期双盲试验评估了790名切除的IIB/C期黑色素瘤患者,他们以2:1(按肿瘤类别分层)随机接受nivolumab 480 mg或安慰剂,每4 12周 月。主要终点是研究者评估的无复发生存率(RFS)。次要终点包括无远处转移生存率(DMFS)和安全性。7.8 最少随访数月后,与安慰剂相比,nivolumab显著改善了RFS(风险比(HR) = 0.42;95%置信区间(CI):0.30-0.59;P
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


