Ultrasonic and Conductometric Studies on l-Aspartic Acid and l-Glutamic Acid in Aqueous Solutions of Sodium Acetate
Abstract
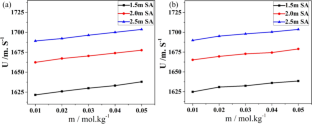
l-Aspartic acid (ASP) and l-glutamic acid (GLU) in aqueous solution of a food additive, sodium acetate (SA) have been taken for acoustic and conductometric studies. The experimental results are analysed to predict and assess the solvation behaviour and types of interactions occurring in these ternary solutions [ASP/GLU + SA + water]. Compressibility parameters like isentropic compressibility and apparent molar isentropic compressibility along with their values at infinite dilution have been computed. Transfer values and ion pair and triplet interactions have been determined. Other thermodynamic parameters like internal pressure, molar free volume, relative association and acoustic impedance, have also been derived from the acoustic data. Specific conductance values measured experimentally led to the calculation of molar conduction of electric charge. Walden product sheds light on structure building or breaking properties of the amino acids. Gibbs thermodynamic functions predict the spontaneity of ion association process. Arrhenius equation is used to calculate activation energy which is indicative of the formation of high-energy transition state required for migration of ions. Analysis of these results establishes the amino acids as water-structure forming agents and predominance of ion–solvent, ion–ion and ion–hydrophilic interactions in the studied systems.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


