Photoinduced, additive- and photosensitizer-free multi-component synthesis of naphthoselenazol-2-amines with air in water†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An atom- and step-economical, efficient and eco-friendly method for constructing naphthoselenazol-2-amines through a visible-light photocatalytic multi-component reaction under aqueous phase conditions is reported. This visible-light-induced reaction proceeded at room temperature under exogenous photosensitizer- and additive-free, neutral and mild conditions with low-cost and abundant elemental selenium as the selenium source, ambient air as the clean oxidant and pure water as the sole solvent.
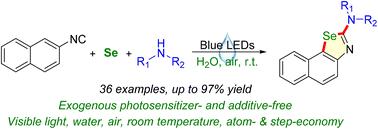
光诱导、无添加剂和光敏剂的多组分合成萘并噻唑-2-胺
报道了一种在水相条件下通过可见光光催化多组分反应构建萘并噻唑-2-胺的原子和步骤经济、高效、环保的方法。这种可见光诱导的反应在室温下在无外源光敏剂和添加剂的中性温和条件下进行,以低成本和丰富的元素硒为硒源,环境空气为清洁氧化剂,纯水为唯一溶剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


