Study on the fluorination of 1, 2-O-isopropylidene D-pentofuranose with DAST and its scalable application
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The research focuses on the inversion of configuration at C3 of 1,2-O-isopropylidene-d-arabinofuranoside and d-ribofuranoside promoted by DAST. Both the initial configuration and the presence of C5 acyl group in d-furanosides are the key factors for this inversion due to the formation of cyclic oxonium ion intermediates. This inversion of configuration has been successfully applied to scalable synthesis of d-lyxose and 2′,5′-di-O-benzoyl-3′-fluoro-3′-deoxy-β-d-ribofuranosyl-7-halogen-7-deazapurine.
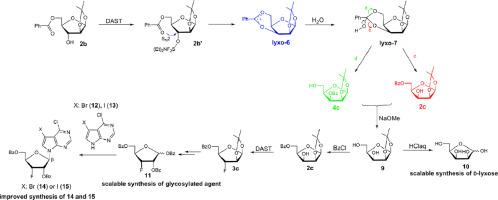
DAST氟化1,2-O-异亚丙基-D-苯并呋喃酮的研究及其应用
研究了DAST促进1,2-O-异亚丙基-d-阿拉伯呋喃糖苷和d-呋喃核糖糖苷C3位构型的反转。由于环状氧鎓离子中间体的形成,d-呋喃糖苷中的初始构型和C5酰基的存在都是这种转化的关键因素。这种构型的反转已成功应用于d-赖氨酸酶和2′,5′-二-O-苯甲酰基-3′-氟-3′-脱氧-β-d-呋喃核糖基-7-卤代-7-脱氮嘌呤的可扩展合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


