Dexmedetomidine alleviates cognitive impairment by promoting hippocampal neurogenesis via BDNF/TrkB/CREB signaling pathway in hypoxic–ischemic neonatal rats
Abstract
Aims
Dexmedetomidine (DEX) has been reported to alleviate hypoxic–ischemic brain damage (HIBD) in neonates. This study aimed to investigate whether DEX improves cognitive impairment by promoting hippocampal neurogenesis via the BDNF/TrkB/CREB signaling pathway in neonatal rats with HIBD.
Methods
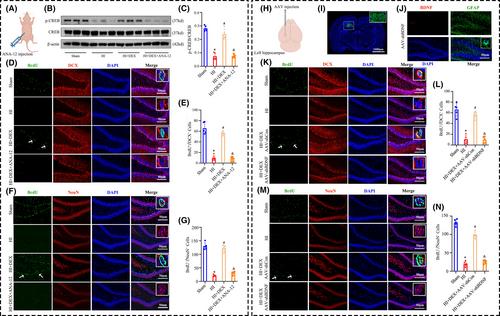
HIBD was induced in postnatal day 7 rats using the Rice-Vannucci method, and DEX (25 μg/kg) was administered intraperitoneally immediately after the HIBD induction. The BDNF/TrkB/CREB pathway was regulated by administering the TrkB receptor antagonist ANA-12 through intraperitoneal injection or by delivering adeno-associated virus (AAV)-shRNA-BDNF via intrahippocampal injection. Western blot was performed to measure the levels of BDNF, TrkB, and CREB. Immunofluorescence staining was utilized to identify the polarization of astrocytes and evaluate the levels of neurogenesis in the dentate gyrus of the hippocampus. Nissl and TTC staining were performed to evaluate the extent of neuronal damage. The MWM test was conducted to evaluate spatial learning and memory ability.
Results
The levels of BDNF and neurogenesis exhibited a notable decrease in the hippocampus of neonatal rats after HIBD, as determined by RNA-sequencing technology. Our results demonstrated that treatment with DEX effectively increased the protein expression of BDNF and the phosphorylation of TrkB and CREB, promoting neurogenesis in the dentate gyrus of the hippocampus in neonatal rats with HIBD. Specifically, DEX treatment significantly augmented the expression of BDNF in hippocampal astrocytes, while decreasing the proportion of detrimental A1 astrocytes and increasing the proportion of beneficial A2 astrocytes in neonatal rats with HIBD. Furthermore, inhibiting the BDNF/TrkB/CREB pathway using either ANA-12 or AAV-shRNA-BDNF significantly counteracted the advantageous outcomes of DEX on hippocampal neurogenesis, neuronal survival, and cognitive improvement.
Conclusions
DEX promoted neurogenesis in the hippocampus by activating the BDNF/TrkB/CREB pathway through the induction of polarization of A1 astrocytes toward A2 astrocytes, subsequently mitigating neuronal damage and cognitive impairment in neonates with HIBD.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


