Structural and functional diversity of type IV secretion systems
IF 69.2
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Considerable progress has been made in recent years in the structural and molecular biology of type IV secretion systems in Gram-negative bacteria. The latest advances have substantially improved our understanding of the mechanisms underlying the recruitment and delivery of DNA and protein substrates to the extracellular environment or target cells. In this Review, we aim to summarize these exciting structural and molecular biology findings and to discuss their functional implications for substrate recognition, recruitment and translocation, as well as the biogenesis of extracellular pili. We also describe adaptations necessary for deploying a breadth of processes, such as bacterial survival, host–pathogen interactions and biotic and abiotic adhesion. We highlight the functional and structural diversity that allows this extremely versatile secretion superfamily to function under different environmental conditions and in different bacterial species. Additionally, we emphasize the importance of further understanding the mechanism of type IV secretion, which will support us in combating antimicrobial resistance and treating type IV secretion system-related infections. In this Review, Costa and colleagues summarize the current knowledge of type IV secretion system functioning in Gram-negative bacteria, with a focus on their architectures and adaptations for specialized functions. They also explore the biogenesis pathways and spatial localization of type IV secretion systems.
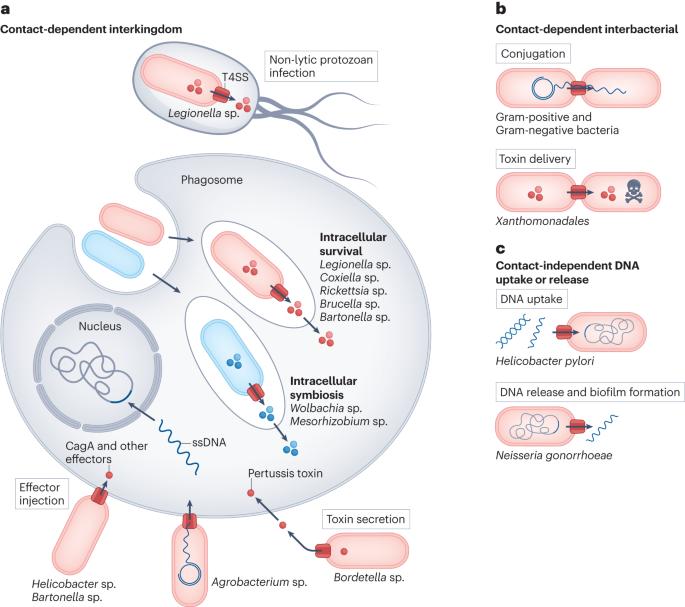
IV型分泌系统的结构和功能多样性。
近年来,在革兰氏阴性菌IV型分泌系统的结构和分子生物学方面取得了相当大的进展。最新进展大大提高了我们对DNA和蛋白质底物募集和递送到细胞外环境或靶细胞的机制的理解。在这篇综述中,我们旨在总结这些令人兴奋的结构和分子生物学发现,并讨论它们对底物识别、募集和易位以及细胞外菌毛的生物发生的功能意义。我们还描述了部署广泛过程所需的适应,如细菌存活、宿主-病原体相互作用以及生物和非生物粘附。我们强调了功能和结构的多样性,使这个功能极其多样的分泌超家族能够在不同的环境条件下和不同的细菌物种中发挥作用。此外,我们强调进一步了解IV型分泌机制的重要性,这将支持我们对抗抗微生物耐药性和治疗IV型分泌系统相关感染。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Microbiology
生物-微生物学
CiteScore
74.00
自引率
0.50%
发文量
149
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Microbiology, our goal is to become the leading source of reviews and commentaries for the scientific community we cater to. We are dedicated to publishing articles that are not only authoritative but also easily accessible, supplementing them with clear and concise figures, tables, and other visual aids. Our objective is to offer an unparalleled service to authors, referees, and readers, and we continuously strive to maximize the usefulness and impact of each article we publish. With a focus on Reviews, Perspectives, and Comments spanning the entire field of microbiology, our wide scope ensures that the work we feature reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


