Pd-Catalyzed Regioselective Cyclopropanation of 2-Substituted 1,3-Dienes
IF 3.3
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A Pd-catalyzed 3,4-regioselective cyclopropanation of 2-substituted 1,3-dienes by decomposition of diazo esters is reported. The vinylcyclopropanes generated are isolated in practical chemical yields with high levels of regioselectivity but low diastereoselectivity. The system operates under mild reaction conditions, is scalable, and tolerates various sensitive functional groups. A series of original postcatalytic derivatizations is presented to highlight the synthetic potential of the catalytic method.
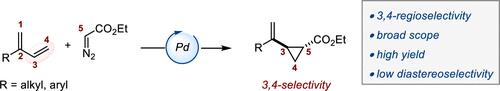
钯催化2-取代-1,3-二烯的区域选择性环丙烷化反应。
报道了钯催化重氮酯分解2-取代-1,3-二烯的3,4-焦选择性环丙烷化反应。所产生的乙烯基环丙烷以实际化学产率分离,具有高水平的区域选择性但低的非对映选择性。该系统在温和的反应条件下运行,可扩展,并耐受各种敏感官能团。介绍了一系列原始的后催化衍生反应,以突出催化方法的合成潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Organic & Inorganic Au
有机化学、无机化学-
CiteScore
4.10
自引率
0.00%
发文量
0
期刊介绍:
ACS Organic & Inorganic Au is an open access journal that publishes original experimental and theoretical/computational studies on organic organometallic inorganic crystal growth and engineering and organic process chemistry. Short letters comprehensive articles reviews and perspectives are welcome on topics that include:Organic chemistry Organometallic chemistry Inorganic Chemistry and Organic Process Chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


