Syntheses of (+)-costic acid and structurally related eudesmane sesquiterpenoids and their biological evaluations as acaricidal agents against Varroa destructor.
IF 1.5
4区 农林科学
Q2 ENTOMOLOGY
引用次数: 0
Abstract
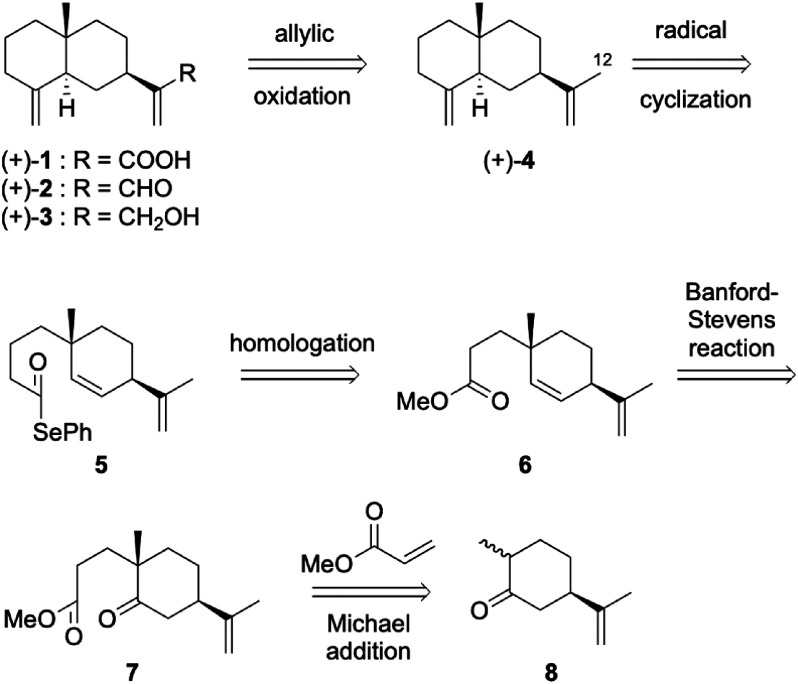
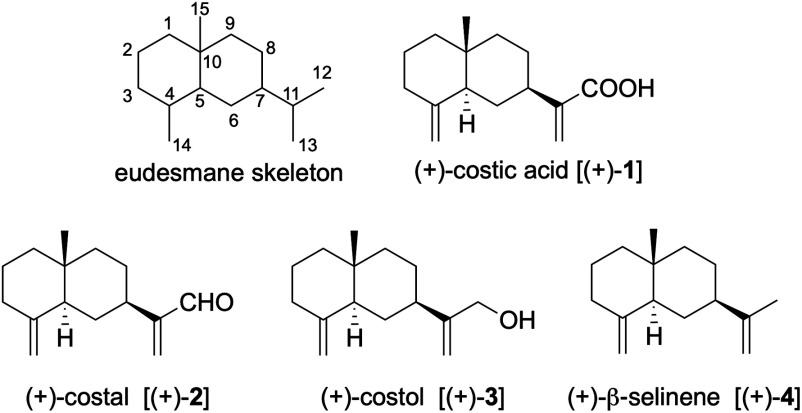
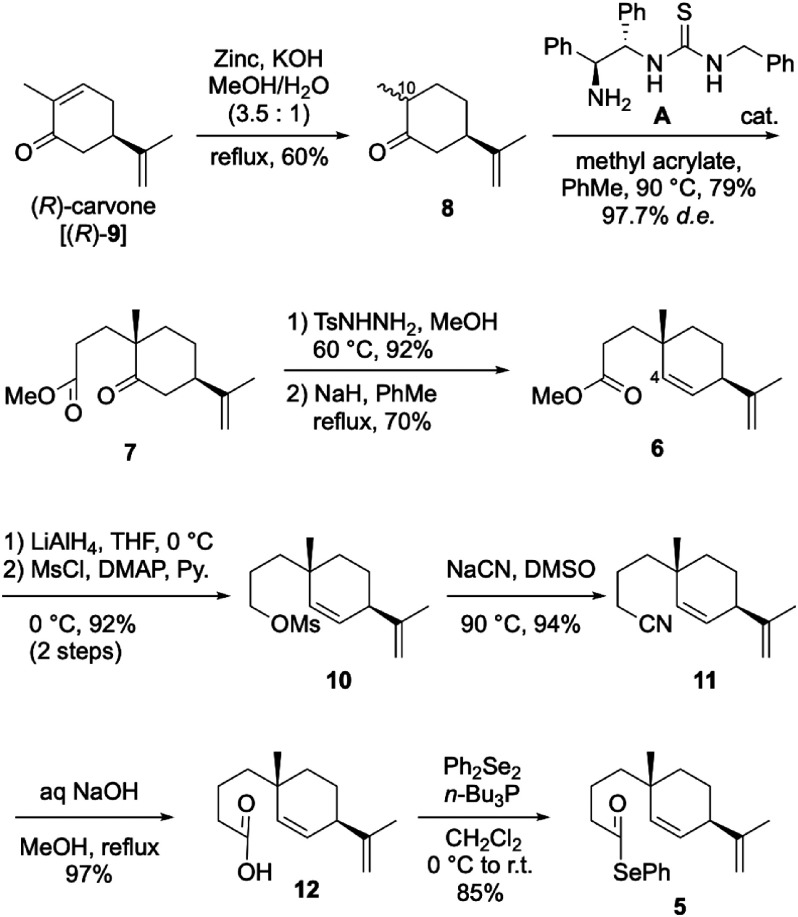
Synthesis of (+)-costic acid, isolated from Dittrichia viscosa (L.) W. Greuter as a natural acaricidal sesquiterpenoid, was achieved in 16 steps from (R)-carvone with an overall yield of 4.8%, involving the radical cyclization of selenoester to construct a decalone framework as the key step. Other structurally related natural products, (+)-costal, (+)-costol, and (+)-β-selinene, were also synthesized. The acaricidal activities of these four natural products and some synthetic intermediates were also evaluated against Varroa destructor. Among them, (+)-costal especially exhibited potent acaricidal activity.
(+)-costic acid和结构相关的真结烷倍半萜类化合物的合成及其作为灭螨剂的生物学评价。
以(R)-香芹酮为原料,经16步反应合成了从粘氏Ditrichia viscosa(L.)W.Greuter中分离的天然杀螨倍半萜类化合物(+)-costic acid,总收率4.8%,关键步骤是硒醚的自由基环化以构建十氢萘骨架。还合成了其他结构相关的天然产物,(+)-costal、(+)-costol和(+)β-selinene。还对这四种天然产物和一些合成中间体的杀螨活性进行了评价。其中(+)-costal表现出较强的杀螨活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Pesticide Science
农林科学-昆虫学
CiteScore
4.30
自引率
4.20%
发文量
28
审稿时长
18-36 weeks
期刊介绍:
The Journal of Pesticide Science publishes the results of original research regarding the chemistry and biochemistry of pesticides including bio-based materials. It also covers their metabolism, toxicology, environmental fate and formulation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


