Analytical assay validation for acute myeloid leukemia measurable residual disease assessment by multiparametric flow cytometry
Abstract
Background
Measurable residual disease (MRD) assessed by multiparametric flow cytometry (MFC) has gained importance in clinical decision-making for acute myeloid leukemia (AML) patients. However, complying with the recent In Vitro Diagnostic Regulations (IVDR) in Europe and Food and Drug Administration (FDA) guidance in the United States requires rigorous validation prior to their use in investigational clinical trials and diagnostics. Validating AML MRD-MFC assays poses challenges due to the unique underlying disease biology and paucity of patient specimens. In this study, we describe an experimental framework for validation that meets regulatory expectations.
Methods
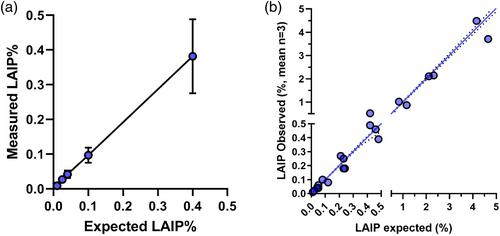
Our validation efforts focused on evaluating assay accuracy, analytical specificity, analytical and functional sensitivity (limit of blank (LoB), detection (LLoD) and quantitation (LLoQ)), precision, linearity, sample/reagent stability and establishing the assay background frequencies.
Results
Correlation between different MFC methods was highly significant (r = 0.99 for %blasts and r = 0.93 for %LAIPs). The analysis of LAIP specificity accurately discriminated from negative control cells. The assay demonstrated a LoB of 0.03, LLoD of 0.04, and LLoQ of 0.1%. Precision experiments yielded highly reproducible results (Coefficient of Variation <20%). Stability experiments demonstrated reliable measurement of samples up to 96 h from collection. Furthermore, the reference range of LAIP frequencies in non-AML patients was below 0.1%, ranging from 0.0% to 0.04%.
Conclusion
In this manuscript, we present the validation of an AML MFC-MRD assay using BM/PB patient specimens, adhering to best practices. Our approach is expected to assist other laboratories in expediting their validation activities to fulfill recent health authority guidelines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


