Telomere dysfunction in Tert knockout mice delays BrafV600E-induced melanoma development
IF 5.7
2区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Telomerase activation is a crucial step in melanomagenesis, often occurring because of ultraviolet radiation (UVR)-induced mutations at the telomerase gene (TERT) promoter and rendering TERT transcription in response to the activated Raf-MAP kinase pathway by BRAFV600E mutation. Due to the excessively long telomeres in mice, this process does not occur during melanomagenesis in mouse models. To investigate the impact of telomere dysfunction on melanomagenesis, BrafV600E was induced in generations 1 and 4 (G1 and G4) of Tert-/- mice. Our findings revealed that, regardless of UVR exposure, melanoma development was delayed in G4 mice, which had shorter telomeres compared to G1 and wild-type C57BL/6J (G0) mice. Moreover, many G4 tumors displayed an accumulation of excessive DNA damage, as evidenced by increased γH2A.X staining. Tumors from UVR-exposed mice exhibited elevated p53 protein expression. Cultured tumor cells isolated from G4 mice displayed abundant chromosomal fusions and rearrangements, indicative of telomere dysfunction in these cells. Additionally, tumor cells derived from UVB-exposed mice exhibited constitutively elevated expression of mutant p53 proteins, suggesting that p53 was a target of UVB-induced mutagenesis. Taken together, our findings suggest that telomere dysfunction hampers melanomagenesis, and targeting telomere crisis-mediated genomic instability may hold promise for the prevention and treatment of melanoma.
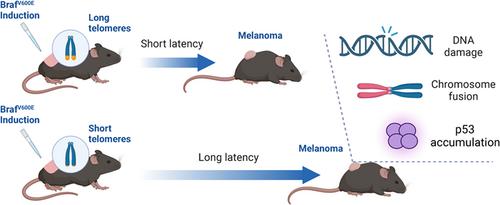
Tert敲除小鼠的端粒功能障碍延迟了BrafV600E诱导的黑色素瘤的发展。
端粒酶激活是黑色素瘤的一个关键步骤,通常发生在紫外线辐射(UVR)诱导的端粒酶基因(TERT)启动子突变,并通过BRAFV600E突变使TERT转录响应激活的Raf-MAP激酶途径。由于小鼠的端粒过长,在小鼠模型的黑色素瘤形成过程中不会发生这种过程。为了研究端粒功能障碍对黑色素瘤的影响,在Tert-/-小鼠的第1代和第4代(G1和G4)中诱导BrafV600E。我们的研究结果表明,无论紫外线照射如何,G4小鼠的黑色素瘤发展都会延迟,与G1和野生型C57BL/6J(G0)小鼠相比,G4小鼠具有更短的端粒。此外,许多G4肿瘤表现出过度DNA损伤的积累,如γH2A.X染色增加所证明的。UVR暴露小鼠的肿瘤表现出p53蛋白表达升高。从G4小鼠分离的培养的肿瘤细胞显示出大量的染色体融合和重排,表明这些细胞中的端粒功能障碍。此外,来源于暴露于UVB的小鼠的肿瘤细胞表现出突变型p53蛋白的组成性表达升高,这表明p53是UVB诱导突变的靶点。总之,我们的研究结果表明,端粒功能障碍阻碍了黑色素瘤的发生,靶向端粒危机介导的基因组不稳定性可能有望预防和治疗黑色素瘤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
13.40
自引率
3.10%
发文量
460
审稿时长
2 months
期刊介绍:
The International Journal of Cancer (IJC) is the official journal of the Union for International Cancer Control—UICC; it appears twice a month. IJC invites submission of manuscripts under a broad scope of topics relevant to experimental and clinical cancer research and publishes original Research Articles and Short Reports under the following categories:
-Cancer Epidemiology-
Cancer Genetics and Epigenetics-
Infectious Causes of Cancer-
Innovative Tools and Methods-
Molecular Cancer Biology-
Tumor Immunology and Microenvironment-
Tumor Markers and Signatures-
Cancer Therapy and Prevention

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


