α-Ketoglutarate–Dependent KDM6 Histone Demethylases and Interferon-Stimulated Gene Expression in Lupus
Abstract
Objective
We aimed to investigate the hypothesis that interferon (IFN)–stimulated gene (ISG) expression in systemic lupus erythematosus (SLE) monocytes is linked to changes in metabolic reprogramming and epigenetic regulation of ISG expression.
Methods
Monocytes from healthy volunteers and patients with SLE at baseline or following IFNα treatment were analyzed by extracellular flux analysis, proteomics, metabolomics, chromatin immunoprecipitation, and gene expression. The histone demethylases KDM6A/B were inhibited using glycogen synthase kinase J4 (GSK-J4). GSK-J4 was tested in pristane and resiquimod (R848) models of IFN-driven SLE.
Results
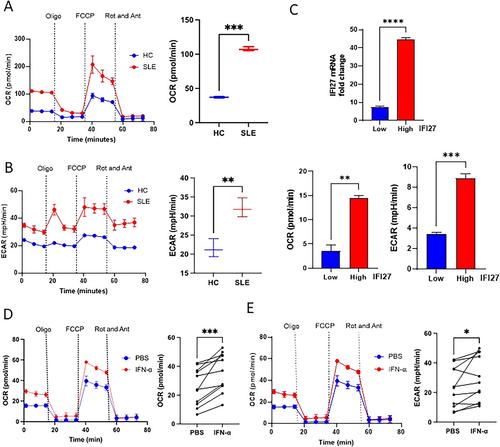
SLE monocytes had enhanced rates of glycolysis and oxidative phosphorylation compared to healthy control monocytes, as well as increased levels of isocitrate dehydrogenase and its product, α-ketoglutarate (α-KG). Because α-KG is a required cofactor for histone demethylases KDM6A and KDM6B, we hypothesized that IFNα may be driving “trained immune” responses through altering histone methylation. IFNα priming (day 1) resulted in a sustained increase in the expression of ISGs in primed cells (day 5) and enhanced expression on restimulation with IFNα. Importantly, decreased H3K27 trimethylation was observed at the promoters of ISGs following IFNα priming. Finally, GSK-J4 (KDM6A/B inhibitor) resulted in decreased ISG expression in SLE patient monocytes, as well as reduced autoantibody production, ISG expression, and kidney pathology in R848-treated BALB/c mice.
Conclusion
Our study suggests long-term IFNα exposure alters the epigenetic regulation of ISG expression in SLE monocytes via changes in immunometabolism, a mechanism reflecting trained immunity to type I IFN. Importantly, it opens the possibility that targeting histone-modifying enzymes, such as KDM6A/B, may reduce IFN responses in SLE.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


