MAFLD fibrosis score: Using routine measures to identify advanced fibrosis in metabolic-associated fatty liver disease
Abstract
Background
Early screening may prevent fibrosis progression in metabolic-associated fatty liver disease (MAFLD).
Aims
We developed and validated MAFLD fibrosis score (MFS) for identifying advanced fibrosis (≥F3) among MAFLD patients.
Methods
This cross-sectional, multicentre study consecutively recruited MAFLD patients receiving tertiary care (Malaysia as training cohort [n = 276] and Hong Kong and Wenzhou as validation cohort [n = 431]). Patients completed liver biopsy, vibration-controlled transient elastography (VCTE), and clinical and laboratory assessment within 1 week. We used machine learning to select ‘highly important’ predictors of advanced fibrosis, followed by backward stepwise regression to construct MFS formula.
Results
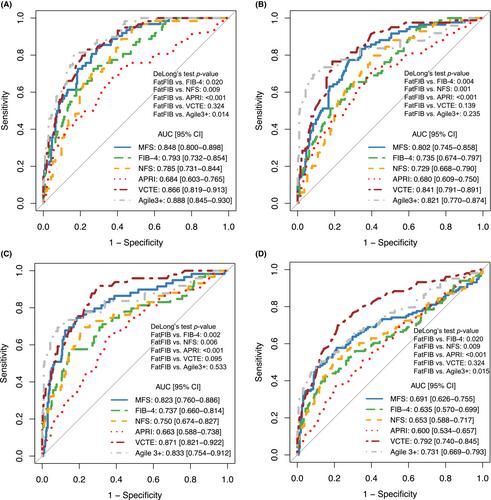
MFS was composed of seven variables: age, body mass index, international normalised ratio, aspartate aminotransferase, gamma-glutamyl transpeptidase, platelet count, and history of type 2 diabetes. MFS demonstrated an area under the receiver-operating characteristic curve of 0.848 [95% CI 0.800–898] and 0.823 [0.760–0.886] in training and validation cohorts, significantly higher than aminotransferase-to-platelet ratio index (0.684 [0.603–0.765], 0.663 [0.588–0.738]), Fibrosis-4 index (0.793 [0.735–0.854], 0.737 [0.660–0.814]), and non-alcoholic fatty liver disease fibrosis score (0.785 [0.731–0.844], 0.750 [0.674–0.827]) (DeLong's test p < 0.05). MFS could include 92.3% of patients using dual cut-offs of 14 and 15, with a correct prediction rate of 90.4%, resulting in a larger number of patients with correct diagnosis compared to other scores. A two-step MFS-VCTE screening algorithm demonstrated positive and negative predictive values and overall diagnostic accuracy of 93.4%, 89.5%, and 93.2%, respectively, with only 4.0% of patients classified into grey zone.
Conclusion
MFS outperforms conventional non-invasive scores in predicting advanced fibrosis, contributing to screening in MAFLD patients.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


