A new cell-free therapeutic strategy for liver regeneration: Human placental mesenchymal stem cell-derived extracellular vesicles.
IF 6.7
1区 工程技术
Q1 CELL & TISSUE ENGINEERING
Journal of Tissue Engineering
Pub Date : 2022-10-20
eCollection Date: 2022-01-01
DOI:10.1177/20417314221132093
引用次数: 3
Abstract
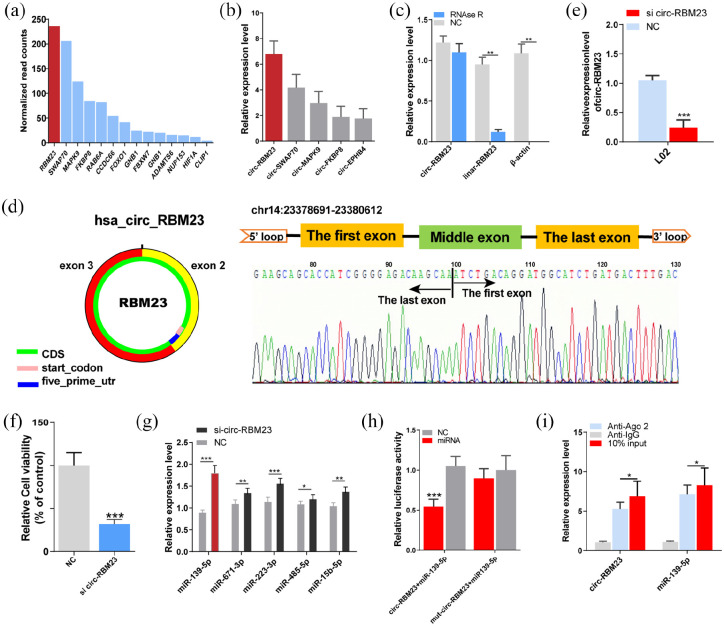
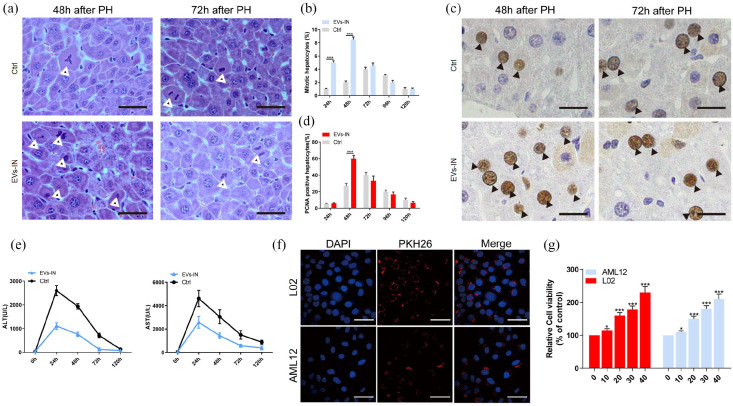
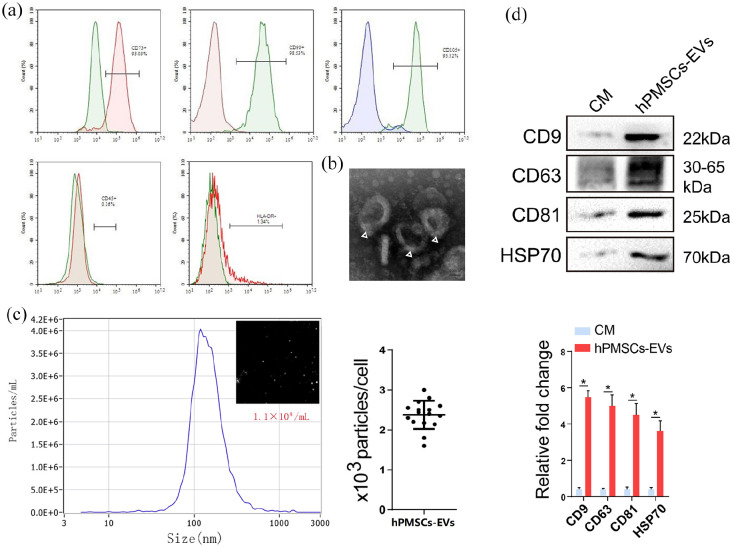
Mesenchymal stem cells (MSCs) have potential role in organ regeneration therapy. Previous work indicating that MSCs confer protection against liver disease. Here, we aimed to determine the potential application in liver regeneration of human placenta-derived MSCs extracellular vesicles (hPMSCs-EVs) via experimental hepatectomy. hPMSCs-EVs were administered intravenously 24 h before 70% partial hepatectomy, the specific composition of hPMSCs-EVs was identified by sequencing and validated by the quantitative polymerase chain reaction, including circ-RBM23. The role of circ-RBM23 in L02 cell was evaluated and it was found that circ-RBM23 knockdown inhibited L02 cell proliferation both in vitro and in vivo. The competing endogenous RNA function of circ-RBM23 was evaluated by the RNA immunoprecipitation assay and found that circ-RBM23 shares miRNA response elements with RRM2. Overexpressed circ-RBM23 bound competitively to miR-139-5p, preventing the miRNA-mediated degradation of RRM2, activating the expression of eIF4G and AKT/mTOR, and facilitating liver regeneration. These results indicate that hPMSCs-EVs prevent hepatic dysfunction and improve liver regeneration in vivo and hepatocytes proliferation in vitro, potentially via circ-RBM23 delivery.
一种新的肝再生无细胞治疗策略:人胎盘间充质干细胞来源的细胞外囊泡。
间充质干细胞在器官再生治疗中具有潜在的作用。先前的研究表明间充质干细胞对肝脏疾病具有保护作用。在这里,我们旨在通过实验性肝切除术确定人胎盘来源的间充质干细胞-细胞外囊泡(hpmscs - ev)在肝脏再生中的潜在应用。在70%部分肝切除术前24 h静脉给予hpmscs - ev,通过测序鉴定hpmscs - ev的特异性组成,并通过定量聚合酶链反应验证,包括circ-RBM23。我们对circ-RBM23在L02细胞中的作用进行了评估,发现circ-RBM23敲低可抑制L02细胞的体外和体内增殖。通过RNA免疫沉淀法评估circ-RBM23的竞争内源RNA功能,发现circ-RBM23与RRM2共享miRNA应答元件。过表达的circ-RBM23与miR-139-5p竞争性结合,阻止mirna介导的RRM2降解,激活eIF4G和AKT/mTOR的表达,促进肝脏再生。这些结果表明,hpmscs - ev可能通过circ-RBM23递送,在体内预防肝功能障碍,改善肝再生和体外肝细胞增殖。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Tissue Engineering
Engineering-Biomedical Engineering
CiteScore
11.60
自引率
4.90%
发文量
52
审稿时长
12 weeks
期刊介绍:
The Journal of Tissue Engineering (JTE) is a peer-reviewed, open-access journal dedicated to scientific research in the field of tissue engineering and its clinical applications. Our journal encompasses a wide range of interests, from the fundamental aspects of stem cells and progenitor cells, including their expansion to viable numbers, to an in-depth understanding of their differentiation processes. Join us in exploring the latest advancements in tissue engineering and its clinical translation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


