Cationic polymer-in-salt electrolytes for fast metal ion conduction and solid-state battery applications
IF 8.9
2区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
Environmental Science & Technology Letters Environ.
Pub Date : 2022-07-28
DOI:10.1038/s41563-022-01319-w
引用次数: 41
Abstract
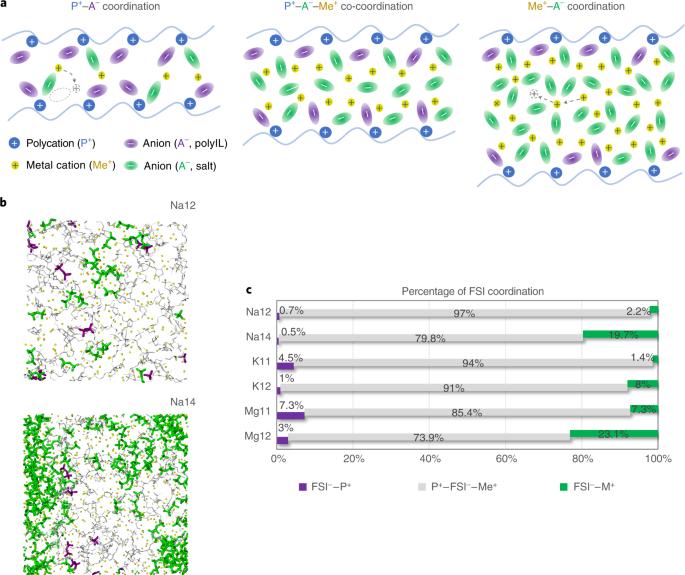
Polymer electrolytes provide a safe solution for future solid-state high-energy-density batteries. Materials that meet the simultaneous requirement of high ionic conductivity and high transference number remain a challenge, in particular for new battery chemistries beyond lithium such as Na, K and Mg. Herein, we demonstrate the versatility of a polymeric ionic liquid (PolyIL) as a polymer solvent to achieve this goal for both Na and K. Using molecular simulations, we predict and elucidate fast alkali metal ion transport in PolyILs through a structural diffusion mechanism in a polymer-in-salt environment, facilitating a high metal ion transference number simultaneously. Experimental validation of these computationally designed Na and K polymer electrolytes shows good ionic conductivities up to 1.0 × 10−3 S cm−1 at 80 °C and a Na+ transference number of ~0.57. An electrochemical cycling test on a Na∣2:1 NaFSI/PolyIL∣Na symmetric cell also demonstrates an overpotential of 100 mV at a current density of 0.5 mA cm−2 and stable long-term Na plating/stripping performance of more than 100 hours. PolyIL-based polymer-in-salt strategies for new solid-state electrolytes thus offer an alternative route to design high-performance next-generation sustainable battery chemistries. Polymer electrolytes provide a safe solution for future solid-state high-energy-density batteries, but combining high ionic conductivity and a high transference number is a challenge. A polymeric ionic liquid used as a polymer solvent is now shown to be promising for both sodium and potassium batteries.
用于快速金属离子传导和固态电池应用的阳离子聚合物盐电解质
聚合物电解质为未来的固态高能量密度电池提供了一种安全的解决方案。同时满足高离子电导率和高转移数量要求的材料仍然是一项挑战,尤其是对于锂以外的新型电池化学物质,如 Na、K 和 Mg。在此,我们展示了聚合物离子液体(PolyIL)作为聚合物溶剂的多功能性,以实现对 Na 和 K 的这一目标。通过分子模拟,我们预测并阐明了聚合物离子液体通过聚合物盐环境中的结构扩散机制实现碱金属离子的快速传输,从而同时促进高金属离子转移数量。对这些通过计算设计的 Na 和 K 聚合物电解质进行的实验验证表明,在 80 °C 时,其离子电导率高达 1.0 × 10-3 S cm-1,Na+离子转移数约为 0.57。在 Na∣2:1 NaFSI/PolyIL∣Na 对称电池上进行的电化学循环测试也表明,在 0.5 mA cm-2 的电流密度下,过电位为 100 mV,Na 的长期稳定电镀/剥离性能超过 100 小时。因此,基于 PolyIL 的盐中聚合物新固态电解质策略为设计高性能的下一代可持续电池化学材料提供了另一条途径。聚合物电解质为未来的固态高能量密度电池提供了一种安全的解决方案,但如何将高离子电导率和高转移数结合起来是一项挑战。目前,一种用作聚合物溶剂的聚合物离子液体已被证明有望用于钠电池和钾电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Science & Technology Letters Environ.
ENGINEERING, ENVIRONMENTALENVIRONMENTAL SC-ENVIRONMENTAL SCIENCES
CiteScore
17.90
自引率
3.70%
发文量
163
期刊介绍:
Environmental Science & Technology Letters serves as an international forum for brief communications on experimental or theoretical results of exceptional timeliness in all aspects of environmental science, both pure and applied. Published as soon as accepted, these communications are summarized in monthly issues. Additionally, the journal features short reviews on emerging topics in environmental science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


