Oncofetal reprogramming in tumour development and progression
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 13
Abstract
Embryonic development is characterized by rapidly dividing cells, cellular plasticity and a highly vascular microenvironment. These features are similar to those of tumour tissue, in that malignant cells are characterized by their ability to proliferate and exhibit cellular plasticity. The tumour microenvironment also often includes immunosuppressive features. Reciprocal communication between various cellular subpopulations enables fetal and tumour tissues to proliferate, migrate and escape immune responses. Fetal-like reprogramming has been demonstrated in the tumour microenvironment, indicating extraordinary cellular plasticity and bringing an additional layer of cellular heterogeneity. More importantly, some of these features are also present during inflammation. This Perspective discusses the similarity between embryogenesis, inflammation and tumorigenesis, and describes the mechanisms of oncofetal reprogramming that enable tumour cells to escape from immune responses, promoting tumour growth and metastasis. Malignant cells show uninhibited proliferation and cellular plasticity, features also reminiscent of embryogenesis. In this Perspective, Sharma and colleagues present their oncofetal ecosystem concept, discuss evidence of oncofetal reprogramming in malignant and non-malignant cells, and debate the therapeutic relevance of these findings.
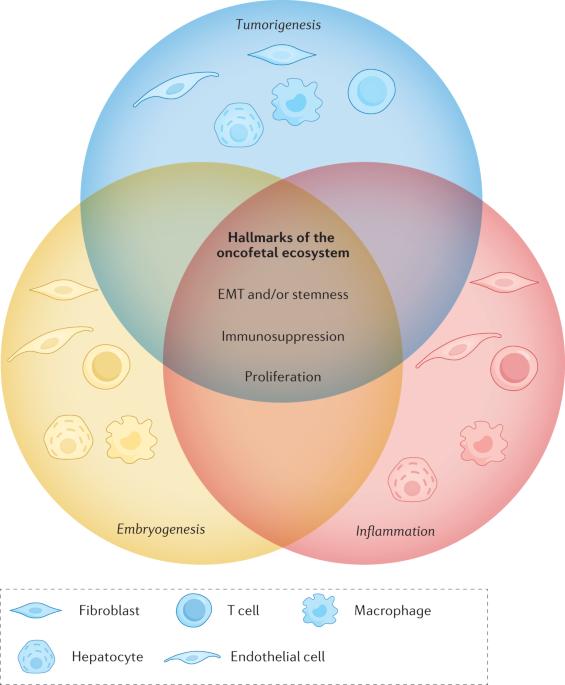
肿瘤发展和恶化过程中的肿瘤胎儿重编程
胚胎发育的特点是细胞快速分裂、细胞可塑性强以及微环境血管丰富。这些特征与肿瘤组织的特征相似,即恶性细胞具有增殖能力和细胞可塑性。肿瘤微环境通常还包括免疫抑制特征。各种细胞亚群之间的相互交流使胎儿和肿瘤组织能够增殖、迁移并逃避免疫反应。肿瘤微环境中的胎儿样重编程已得到证实,这表明细胞具有非凡的可塑性,并带来了额外的细胞异质性。更重要的是,其中一些特征在炎症期间也会出现。本视角探讨了胚胎发生、炎症和肿瘤发生之间的相似性,并描述了使肿瘤细胞逃避免疫反应、促进肿瘤生长和转移的胎盘重编程机制。恶性细胞表现出增殖不受抑制和细胞可塑性,这些特征也让人联想到胚胎发生。在本《视角》中,夏尔马及其同事介绍了他们的胎盘生态系统概念,讨论了恶性和非恶性细胞中胎盘重编程的证据,并讨论了这些发现的治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


