Dual action of ketamine confines addiction liability
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 23
Abstract
Ketamine is used clinically as an anaesthetic and a fast-acting antidepressant, and recreationally for its dissociative properties, raising concerns of addiction as a possible side effect. Addictive drugs such as cocaine increase the levels of dopamine in the nucleus accumbens. This facilitates synaptic plasticity in the mesolimbic system, which causes behavioural adaptations and eventually drives the transition to compulsion1–4. The addiction liability of ketamine is a matter of much debate, in part because of its complex pharmacology that among several targets includes N-methyl-d-aspartic acid (NMDA) receptor (NMDAR) antagonism5,6. Here we show that ketamine does not induce the synaptic plasticity that is typically observed with addictive drugs in mice, despite eliciting robust dopamine transients in the nucleus accumbens. Ketamine nevertheless supported reinforcement through the disinhibition of dopamine neurons in the ventral tegmental area (VTA). This effect was mediated by NMDAR antagonism in GABA (γ-aminobutyric acid) neurons of the VTA, but was quickly terminated by type-2 dopamine receptors on dopamine neurons. The rapid off-kinetics of the dopamine transients along with the NMDAR antagonism precluded the induction of synaptic plasticity in the VTA and the nucleus accumbens, and did not elicit locomotor sensitization or uncontrolled self-administration. In summary, the dual action of ketamine leads to a unique constellation of dopamine-driven positive reinforcement, but low addiction liability. Experiments in mice show that although ketamine has positive reinforcement properties, which are driven by its action on the dopamine system, it does not induce the synaptic plasticity that is typically observed with addiction.
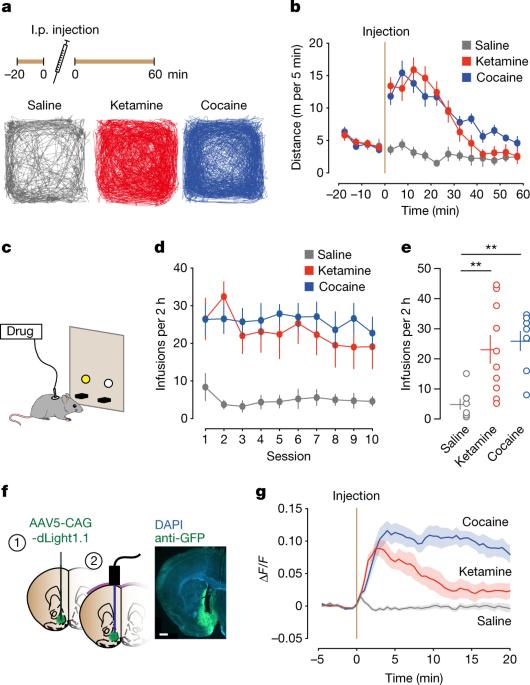
氯胺酮的双重作用限制了成瘾责任
氯胺酮在临床上被用作麻醉剂和速效抗抑郁剂,在娱乐方面则因其解离特性而引起人们对其可能产生的副作用上瘾的担忧。可卡因等成瘾性药物会增加脑核中的多巴胺水平。这促进了间叶系统的突触可塑性,从而导致行为适应,并最终推动向强迫性过渡1-4。氯胺酮的成瘾性是一个备受争议的问题,部分原因是其复杂的药理作用,其中包括N-甲基-d-天冬氨酸(NMDA)受体(NMDAR)拮抗作用5,6。我们在这里发现,尽管氯胺酮能在小鼠的伏隔核中引起强烈的多巴胺瞬时效应,但它并不能诱导小鼠产生通常在成瘾药物中观察到的突触可塑性。然而,氯胺酮通过解除对腹侧被盖区(VTA)多巴胺神经元的抑制,支持了强化作用。这种效应是由 VTA 的 GABA(γ-氨基丁酸)神经元中的 NMDAR 拮抗介导的,但很快就会被多巴胺神经元上的 2 型多巴胺受体终止。多巴胺瞬态的快速离体动力学以及 NMDAR 拮抗作用排除了诱导 VTA 和伏隔核突触可塑性的可能性,也不会引起运动敏感化或失控的自我给药。总之,氯胺酮的双重作用导致了一种独特的多巴胺驱动的正强化,但成瘾性较低。在小鼠身上进行的实验表明,尽管氯胺酮具有正强化特性,而这种特性是由其对多巴胺系统的作用所驱动的,但它并不会诱发通常与成瘾有关的突触可塑性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


