Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 31
Abstract
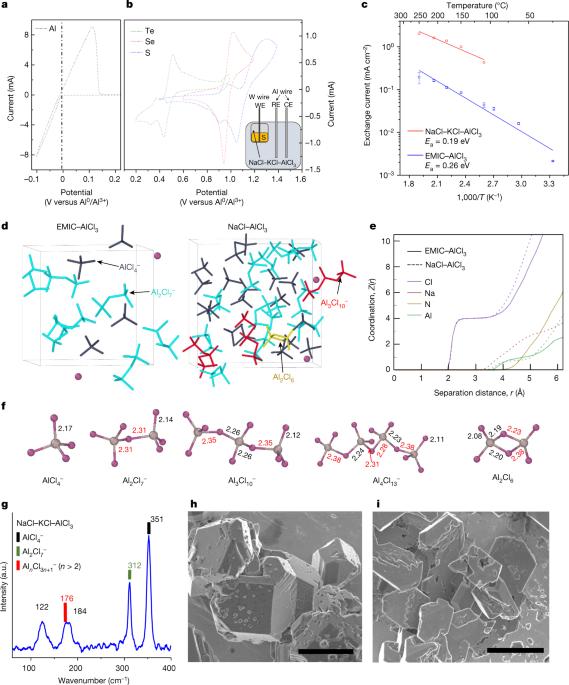
Although batteries fitted with a metal negative electrode are attractive for their higher energy density and lower complexity, the latter making them more easily recyclable, the threat of cell shorting by dendrites has stalled deployment of the technology1,2. Here we disclose a bidirectional, rapidly charging aluminium–chalcogen battery operating with a molten-salt electrolyte composed of NaCl–KCl–AlCl3. Formulated with high levels of AlCl3, these chloroaluminate melts contain catenated AlnCl3n+1– species, for example, Al2Cl7–, Al3Cl10– and Al4Cl13–, which with their Al–Cl–Al linkages confer facile Al3+ desolvation kinetics resulting in high faradaic exchange currents, to form the foundation for high-rate charging of the battery. This chemistry is distinguished from other aluminium batteries in the choice of a positive elemental-chalcogen electrode as opposed to various low-capacity compound formulations3–6, and in the choice of a molten-salt electrolyte as opposed to room-temperature ionic liquids that induce high polarization7–12. We show that the multi-step conversion pathway between aluminium and chalcogen allows rapid charging at up to 200C, and the battery endures hundreds of cycles at very high charging rates without aluminium dendrite formation. Importantly for scalability, the cell-level cost of the aluminium–sulfur battery is projected to be less than one-sixth that of current lithium-ion technologies. Composed of earth-abundant elements that can be ethically sourced and operated at moderately elevated temperatures just above the boiling point of water, this chemistry has all the requisites of a low-cost, rechargeable, fire-resistant, recyclable battery. An aluminium–chalcogen battery operating with a molten-salt electrolyte composed of NaCl–KCl–AlCl3 is presented, which allows rapid charging at up to 200C for hundreds of cycles, and is scalable, fire-resistant and low cost.
耐树枝状短路的快速充电铝钙原电池
尽管装有金属负极的电池因其能量密度高、复杂性低(后者使其更易于回收)而颇具吸引力,但树枝状突起造成的电池短路威胁却阻碍了该技术的应用1,2。在此,我们揭示了一种双向、快速充电的铝-钙原电池,该电池使用由 NaCl-KCl-AlCl3 组成的熔盐电解质。这些氯铝酸盐熔体中含有高含量的 AlCl3,例如 Al2Cl7-、Al3Cl10- 和 Al4Cl13-,它们与 Al-Cl-Al 的连接使 Al3+ 易于脱溶,从而产生高的法拉第交换电流,为电池的高速充电奠定了基础。这种化学电池与其他铝电池的不同之处在于,它选择了元素-钙原正极,而不是各种低容量的化合物配方3-6;它选择了熔盐电解质,而不是会引起高极化的室温离子液体7-12。我们的研究表明,铝和绿原酸之间的多步转换途径允许在高达 200C 的温度下快速充电,电池在极高的充电速率下可承受数百次循环,且不会形成铝枝晶。重要的是,铝硫电池的电池级成本预计不到当前锂离子技术的六分之一。这种化学物质由地球上丰富的元素组成,可以通过道德途径获取,并在略高于水沸点的适度高温下运行,具备了低成本、可充电、耐火、可回收电池的所有必要条件。本文介绍了一种使用由 NaCl-KCl-AlCl3 组成的熔盐电解质的铝-钙原电池,这种电池可以在高达 200C 的温度下快速充电数百次,并且具有可扩展性、耐火性和低成本。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


