Synthesis of a Triphenylphosphinimide-Substituted Silirane as a “Masked” Acyclic Silylene
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 1
Abstract
Phosphinimides are long known as useful ligands in transition metal chemistry, but examples of these in low-valent silicon chemistry are rather rare. Hence, in this work, we report on the implementation of a triphenylphosphinimide moiety as a ligand of a novel silylene that is trapped as a silirane with cyclohexene. By performing activation reactions with B(p-Tol)3, HSiEt3, N2O, and NH3, we demonstrate that the silirane exhibits a silylene-like behavior, making it a "masked" silylene. Furthermore, we treated the silirane with ethylene, propylene, and trans-butene, which led to an olefin exchange. In the case of ethylene and propylene, an additional insertion of the olefin into the silicon-silicon bonds of the respective siliranes could be achieved. As the insertion of trans-butene was not feasible, we surmise that the scope of this reactivity is restricted by the steric demand of the olefin.
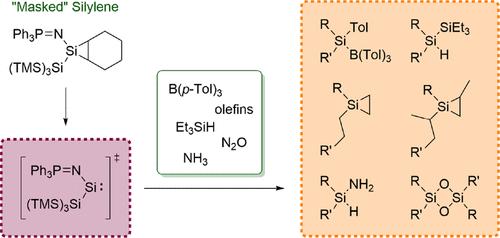
三苯基膦酰亚胺取代硅烷“蒙面”无环硅烯的合成
在过渡金属化学中,磷酰亚胺一直被认为是有用的配体,但在低价硅化学中,它们的例子相当罕见。因此,在这项工作中,我们报道了三苯基膦酰亚胺部分作为一种新型硅烷的配体的实现,这种硅烷与环己烯被捕获为硅烷。通过与B(p-Tol)3, HSiEt3, N2O和NH3进行活化反应,我们证明了硅烷具有类似硅烯的行为,使其成为“掩膜”硅烯。此外,我们用乙烯、丙烯和反式丁烯处理硅烷,导致烯烃交换。在乙烯和丙烯的情况下,可以将烯烃额外插入到各自硅烷的硅-硅键中。由于插入反式丁烯不可行,我们推测这种反应性的范围受到烯烃的空间需求的限制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


