Innate Immune Training with Bacterial Extracts Enhances Lung Macrophage Recruitment to Protect from Betacoronavirus Infection.
IF 3
3区 医学
Q2 IMMUNOLOGY
引用次数: 13
Abstract
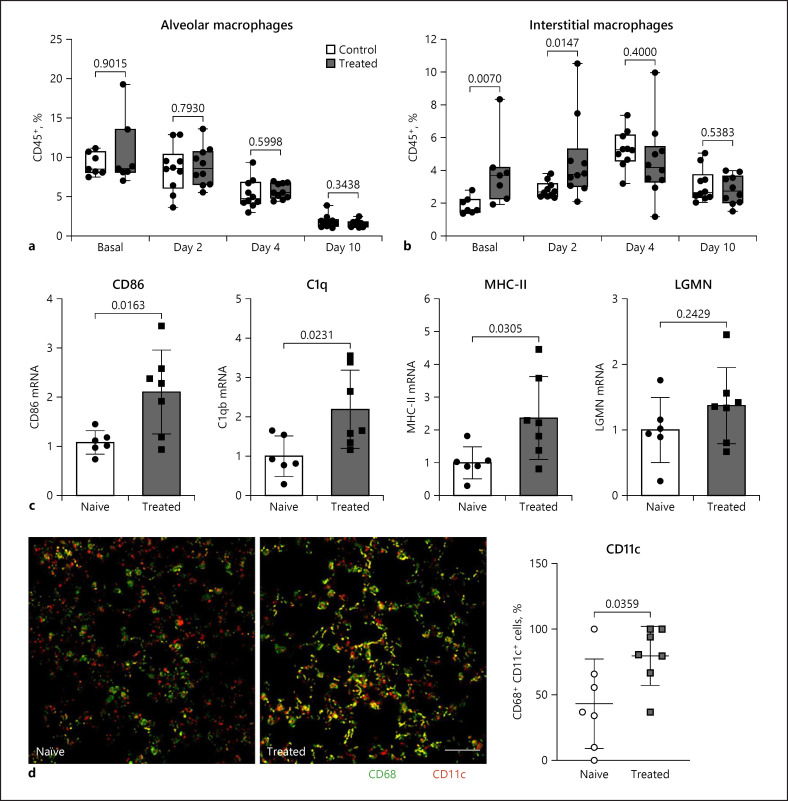
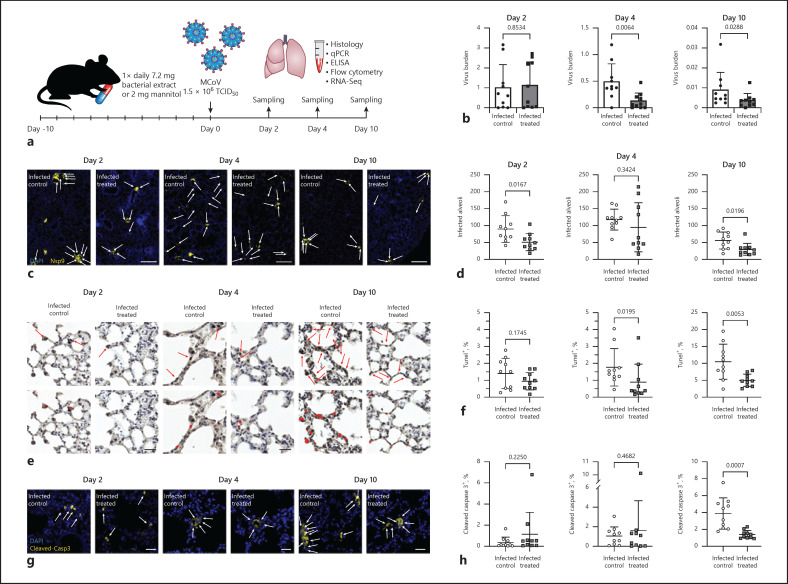
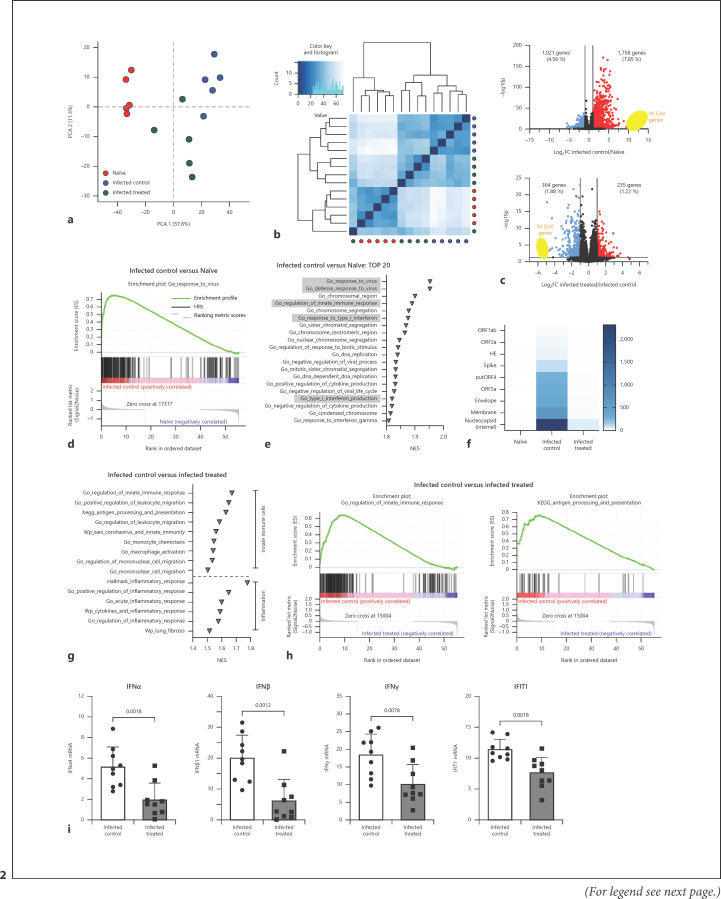
Training of the innate immune system with orally ingested bacterial extracts was demonstrated to have beneficial effects on infection clearance and disease outcome. The aim of our study was to identify cellular and molecular processes responsible for these immunological benefits. We used a murine coronavirus (MCoV) A59 mouse model treated with the immune activating bacterial extract Broncho-Vaxom (BV) OM-85. Tissue samples were analysed with qPCR, RNA sequencing, histology, and flow cytometry. After BV OM-85 treatment, interstitial macrophages accumulated in lung tissue leading to a faster response of type I interferon (IFN) signalling after MCoV infection resulting in overall lung tissue protection. Moreover, RNA sequencing showed that lung tissue from mice receiving BV OM-85 resembled an intermediate stage between healthy and viral infected lung tissue at day 4, indicating a faster return to normal tissue homoeostasis. The pharmacologic effect was mimicked by adoptively transferring naive lung macrophages into lungs from recipient mice before virus infection. The beneficial effect of BV OM-85 was abolished when inhibiting initial type I IFN signalling. Overall, our data suggest that BV OM-85 enhances lung macrophages allowing for a faster IFN response towards a viral challenge as part of the oral-induced innate immune system training.
用细菌提取物进行先天免疫训练可增强肺巨噬细胞募集以保护免受冠状病毒感染。
通过口服细菌提取物训练先天免疫系统被证明对感染清除和疾病结局有有益的影响。我们研究的目的是确定负责这些免疫益处的细胞和分子过程。我们用免疫激活细菌提取物Broncho-Vaxom -85处理小鼠冠状病毒(MCoV) A59小鼠模型。组织样本采用qPCR、RNA测序、组织学和流式细胞术进行分析。BV OM-85治疗后,间质巨噬细胞在肺组织中积累,导致MCoV感染后I型干扰素(IFN)信号传导反应更快,从而整体保护肺组织。此外,RNA测序显示,接受BV OM-85的小鼠肺组织在第4天类似于健康和病毒感染的肺组织之间的中间阶段,表明更快地恢复正常组织稳态。通过在病毒感染前将未成熟的肺巨噬细胞过继转移到受体小鼠的肺中来模拟其药理作用。当抑制初始I型IFN信号传导时,BV OM-85的有益作用被取消。总的来说,我们的数据表明,BV OM-85增强肺巨噬细胞,使IFN对病毒攻击的反应更快,这是口服诱导的先天免疫系统训练的一部分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Innate Immunity
医学-免疫学
CiteScore
10.50
自引率
1.90%
发文量
35
审稿时长
7.5 months
期刊介绍:
The ''Journal of Innate Immunity'' is a bimonthly journal covering all aspects within the area of innate immunity, including evolution of the immune system, molecular biology of cells involved in innate immunity, pattern recognition and signals of ‘danger’, microbial corruption, host response and inflammation, mucosal immunity, complement and coagulation, sepsis and septic shock, molecular genomics, and development of immunotherapies. The journal publishes original research articles, short communications, reviews, commentaries and letters to the editors. In addition to regular papers, some issues feature a special section with a thematic focus.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


