Application of a deep learning-based image analysis and live-cell imaging system for quantifying adipogenic differentiation kinetics of adipose-derived stem/stromal cells.
IF 3.5
4区 生物学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 3
Abstract
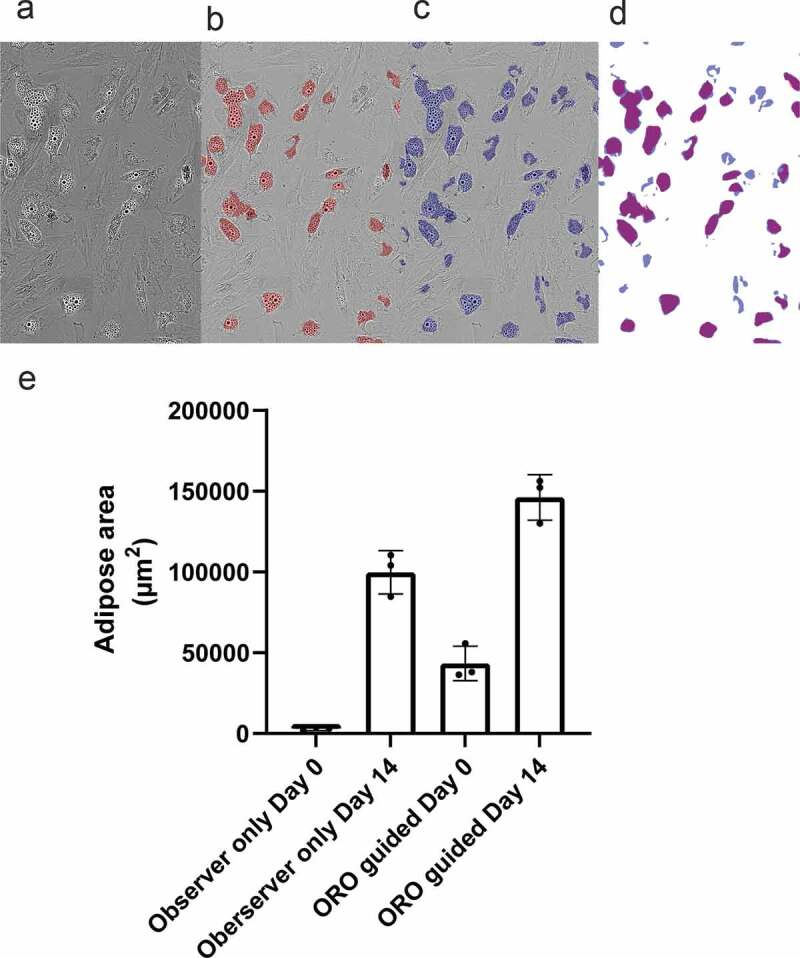
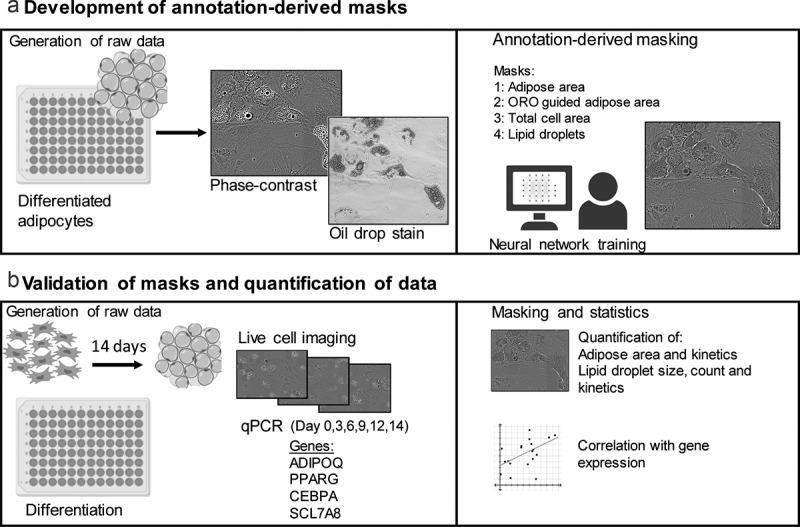
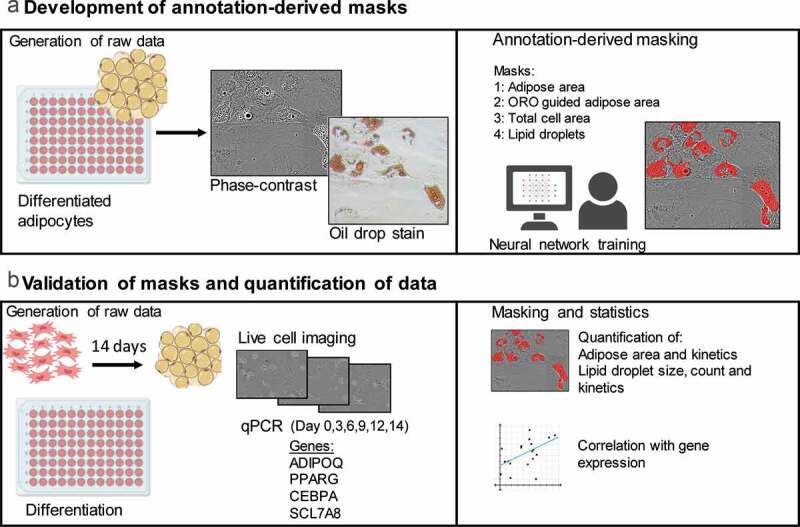
ABSTRACT Quantitative methods for assessing differentiative potency of adipose-derived stem/stromal cells may lead to improved clinical application of this multipotent stem cell, by advancing our understanding of specific processes such as adipogenic differentiation. Conventional cell staining methods are used to determine the formation of adipose areas during adipogenesis as a qualitative representation of adipogenic potency. Staining methods such as oil-red-O are quantifiable using absorbance measurements, but these assays are time and material consuming. Detection methods for cell characteristics using advanced image analysis by machine learning are emerging. Here, live-cell imaging was combined with a deep learning-based detection tool to quantify the presence of adipose areas and lipid droplet formation during adipogenic differentiation of adipose-derived stem/stromal cells. Different detection masks quantified adipose area and lipid droplet formation at different time points indicating kinetics of adipogenesis and showed differences between individual donors. Whereas CEBPA and PPARG expression seems to precede the increase in adipose area and lipid droplets, it might be able to predict expression of ADIPOQ. The applied method is a proof of concept, demonstrating that deep learning methods can be used to investigate adipogenic differentiation and kinetics in vitro using specific detection masks based on algorithm produced from annotation of image data.
应用基于深度学习的图像分析和活细胞成像系统定量脂肪源性干细胞/基质细胞的成脂分化动力学。
定量评估脂肪源性干细胞/基质细胞分化能力的方法,可能会促进我们对脂肪生成分化等特定过程的理解,从而改善这种多能干细胞的临床应用。传统的细胞染色方法用于确定脂肪形成过程中脂肪区域的形成,作为脂肪形成能力的定性代表。染色方法,如油-红- o是可量化使用吸光度测量,但这些分析是时间和材料消耗。利用机器学习进行高级图像分析的细胞特征检测方法正在出现。在这里,活细胞成像与基于深度学习的检测工具相结合,量化脂肪源性干细胞/基质细胞成脂分化过程中脂肪区域的存在和脂滴的形成。不同的检测面罩在不同的时间点量化脂肪面积和脂滴的形成,这表明脂肪形成的动力学,并且在个体供体之间存在差异。而CEBPA和PPARG的表达似乎先于脂肪面积和脂滴的增加,它可能能够预测ADIPOQ的表达。所应用的方法是一个概念证明,表明深度学习方法可以用于研究体外成脂分化和动力学,使用基于图像数据注释生成的算法的特定检测掩模。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Adipocyte
Medicine-Histology
CiteScore
6.50
自引率
3.00%
发文量
46
审稿时长
32 weeks
期刊介绍:
Adipocyte recognizes that the adipose tissue is the largest endocrine organ in the body, and explores the link between dysfunctional adipose tissue and the growing number of chronic diseases including diabetes, hypertension, cardiovascular disease and cancer. Historically, the primary function of the adipose tissue was limited to energy storage and thermoregulation. However, a plethora of research over the past 3 decades has recognized the dynamic role of the adipose tissue and its contribution to a variety of physiological processes including reproduction, angiogenesis, apoptosis, inflammation, blood pressure, coagulation, fibrinolysis, immunity and general metabolic homeostasis. The field of Adipose Tissue research has grown tremendously, and Adipocyte is the first international peer-reviewed journal of its kind providing a multi-disciplinary forum for research focusing exclusively on all aspects of adipose tissue physiology and pathophysiology. Adipocyte accepts high-profile submissions in basic, translational and clinical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


