Alpha-arrestins Aly1/Art6 and Aly2/Art3 regulate trafficking of the glycerophosphoinositol transporter Git1 and impact phospholipid homeostasis
Abstract
Background information
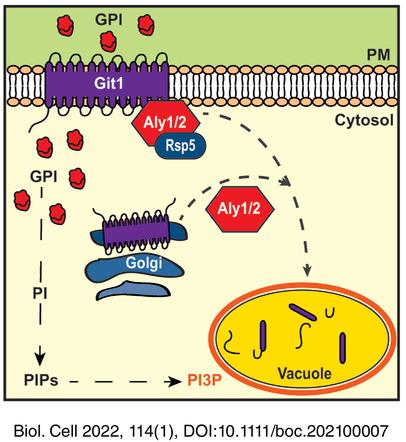
Phosphatidylinositol (PI) is an essential phospholipid, critical to membrane bilayers. The complete deacylation of PI by B-type phospholipases produces intracellular and extracellular glycerophosphoinositol (GPI). Extracellular GPI is transported into the cell via Git1, a member of the Major Facilitator Superfamily of transporters at the yeast plasma membrane. Internalized GPI is degraded to produce inositol, phosphate and glycerol, thereby contributing to these pools. GIT1 gene expression is controlled by nutrient balance, with phosphate or inositol starvation increasing GIT1 expression to stimulate GPI uptake. However, less is known about control of Git1 protein levels or localization.
Results
We find that the α-arrestins, an important class of protein trafficking adaptor, regulate Git1 localization and this is dependent upon their interaction with the ubiquitin ligase Rsp5. Specifically, α-arrestin Aly2 stimulates Git1 trafficking to the vacuole under basal conditions, but in response to GPI-treatment, either Aly1 or Aly2 promote Git1 vacuole trafficking. Cell surface retention of Git1, as occurs in aly1∆ aly2∆ cells, is linked to impaired growth in the presence of exogenous GPI and results in increased uptake of radiolabeled GPI, suggesting that accumulation of GPI somehow causes cellular toxicity. Regulation of α-arrestin Aly1 by the protein phosphatase calcineurin improves steady-state and substrate-induced trafficking of Git1, however, calcineurin plays a larger role in Git1 trafficking beyond regulation of α-arrestins. Interestingly, loss of Aly1 and Aly2 increased phosphatidylinositol-3-phosphate on the limiting membrane of the vacuole, and this was further exacerbated by GPI addition, suggesting that the effect is partially linked to Git1. Loss of Aly1 and Aly2 leads to increased incorporation of inositol label from [3H]-inositol-labelled GPI into PI, confirming that internalized GPI influences PI balance and indicating a role for the a-arrestins in this regulation.
Conclusions
The α-arrestins Aly1 and Aly2 are novel regulators of Git1 trafficking with previously unanticipated roles in controlling phospholipid distribution and balance.
Significance
To our knowledge, this is the first example of α-arrestin regulation of phosphatidyliniositol-3-phosphate levels. In future studies it will be exciting to determine if other α-arrestins similarly alter PI and PIPs to change the cellular landscape.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


