Macrophage IRX3 promotes diet-induced obesity and metabolic inflammation
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 28
Abstract
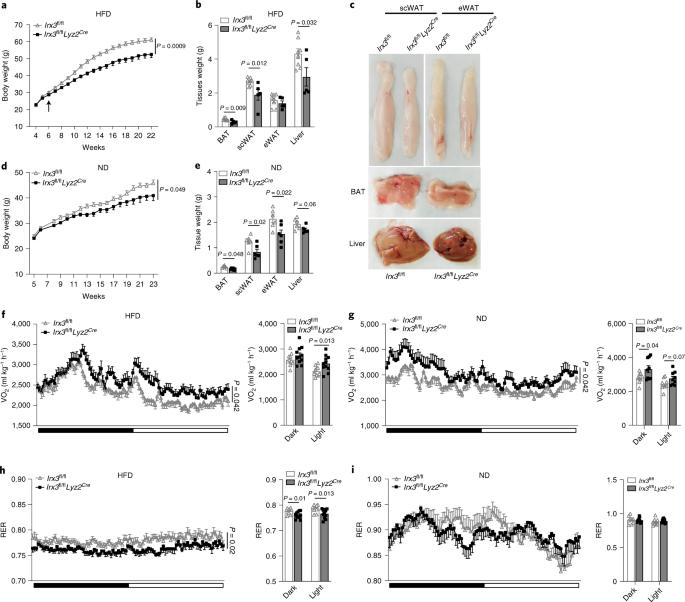
Metabolic inflammation is closely linked to obesity, and is implicated in the pathogenesis of metabolic diseases. FTO harbors the strongest genetic association with polygenic obesity, and IRX3 mediates the effects of FTO on body weight. However, in what cells and how IRX3 carries out this control are poorly understood. Here we report that macrophage IRX3 promotes metabolic inflammation to accelerate the development of obesity and type 2 diabetes. Mice with myeloid-specific deletion of Irx3 were protected against diet-induced obesity and metabolic diseases via increasing adaptive thermogenesis. Mechanistically, macrophage IRX3 promoted proinflammatory cytokine transcription and thus repressed adipocyte adrenergic signaling, thereby inhibiting lipolysis and thermogenesis. JNK1/2 phosphorylated IRX3, leading to its dimerization and nuclear translocation for transcription. Further, lipopolysaccharide stimulation stabilized IRX3 by inhibiting its ubiquitination, which amplified the transcriptional capacity of IRX3. Together, our findings identify a new player, macrophage IRX3, in the control of body weight and metabolic inflammation, implicating IRX3 as a therapeutic target. Adipose tissue macrophages are intimately involved with adipocytes to orchestrate whole-body energy metabolism. Qiu and colleagues show that myeloid-specific deletion of the homeobox protein IRX3 protects against diet-induced obesity, excessive proinflammatory cytokine secretion and metabolic diseases via increasing adaptive thermogenesis.
巨噬细胞 IRX3 促进饮食引起的肥胖和代谢性炎症
代谢性炎症与肥胖密切相关,并与代谢性疾病的发病机制有关。FTO 与多基因肥胖的遗传关系最为密切,而 IRX3 则介导 FTO 对体重的影响。然而,IRX3 在哪些细胞中以及如何发挥这种控制作用却鲜为人知。在这里,我们报告了巨噬细胞 IRX3 促进代谢炎症,从而加速肥胖和 2 型糖尿病的发展。髓系特异性缺失IRX3的小鼠可通过增加适应性产热来防止饮食引起的肥胖和代谢性疾病。从机理上讲,巨噬细胞IRX3促进了促炎细胞因子的转录,从而抑制了脂肪细胞肾上腺素能信号传导,从而抑制了脂肪分解和产热。JNK1/2使IRX3磷酸化,导致其二聚化和核转运转录。此外,脂多糖刺激通过抑制IRX3的泛素化使其稳定,从而增强了IRX3的转录能力。总之,我们的研究结果确定了巨噬细胞IRX3在控制体重和代谢性炎症中的新角色,并将IRX3作为一个治疗靶点。脂肪组织巨噬细胞与脂肪细胞密切相关,共同协调全身能量代谢。Qiu及其同事的研究表明,髓系特异性缺失homeobox蛋白IRX3可通过增加适应性产热,防止饮食引起的肥胖、促炎细胞因子分泌过多和代谢性疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


