Murine cancer cachexia models replicate elevated catabolic pembrolizumab clearance in humans
Abstract
Background
Monoclonal antibody (mAb) immune checkpoint inhibitor (ICI) therapies have dramatically impacted oncology this past decade. However, only about one-third of patients respond to treatment, and biomarkers to predict responders are lacking. Recent ICI clinical pharmacology data demonstrate high baseline drug clearance (CL0) significantly associates with shorter overall survival, independent of ICI exposure, in patients receiving ICI mAb therapies. This suggests CL0 may predict outcomes from ICI therapy, and cachectic signalling may link elevated CL0 and poor response. Our aim was to determine if mouse models of cancer cachexia will be useful for studying these phenomena and their underlying mechanisms.
Methods
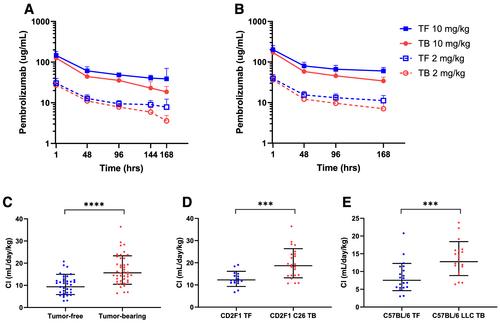
We evaluated pembrolizumab CL in the C26 and Lewis lung carcinoma mouse models of cancer cachexia. A single treatment of vehicle or pembrolizumab, at a dose of 2 or 10 mg/kg, was administered intravenously by tail vein injection. Pembrolizumab was quantified by an ELISA in serial plasma samples, and FcRn gene (Fcgrt) expression was assessed in liver using real-time quantitative reverse transcription PCR. Non-compartmental and mixed-effects pharmacokinetics analyses were performed.
Results
We observed higher pembrolizumab CL0 and decreased Fcgrt expression in whole liver tissue from tumour-bearing vs. tumour-free mice. In multivariate analysis, presence of tumour, total murine IgG, muscle weight and Fcgrt expression were significant covariates on CL, and total murine IgG was a significant covariate on V1 and Q.
Conclusions
These data demonstrate increases in catabolic clearance of monoclonal antibodies observed in humans can be replicated in cachectic mice, in which Fcgrt expression is also reduced. Notably, FcRn activity is essential for proper antigen presentation and antitumour immunity, which may permit the study of cachexia's impact on FcRn-mediated clearance and efficacy of ICI therapies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


